Gain-of-function mutations in the calcium-sensing receptor (CaSR) result in congenital hypoparathyroidism, characterized by suppressed parathyroid hormone (PTH) secretion, hypocalcemia, hyperphosphatemia and hypercalciuria. Managing hypocalcemia in these patients is challenging, as conventional therapy with calcium and active vitamin D analog supplementation often exacerbates renal complications. Teriparatide, a recombinant PTH (1-34) analog, has been approved as an alternative treatment in adult patient. However, data on its prolonged use in pediatric patients remain limited.
We present a pediatric case of teriparatide therapy for hypoparathyroidism due to a de novo CaSR mutation, spanning more than 10 years from infancy into late childhood. In this case, the importance of adequate supplementation of native vitamin D and calcium was illustrated by the drastic reduction in teriparatide dosage requirement. In this case, satisfactory growth without major side effects and static nephrocalcinosis were demonstrated during long-term teriparatide therapy.
Further studies are required to define optimal PTH analog dosage in pediatric patient, to evaluate extended therapeutic outcomes, and explore real-time calcium monitoring strategies to refine management in congenital hypoparathyroidism.
Calcium-sensing receptor defect, Hypoparathyroidism, Recombinant parathyroid hormone analog, Vitamin D, Teriparatide
Gain-of-function mutations in the calcium-sensing receptor (CaSR) cause congenital hypoparathyroidism by inappropriately suppressing parathyroid hormone (PTH) secretion, resulting in hypocalcemia, hyperphosphatemia, and hypercalciuria [1]. Conventional management with calcium supplement and active vitamin D analog often exacerbates hypercalciuria, increasing the risk of nephrocalcinosis and renal impairment [2]. This presents a therapeutic challenge, particularly in pediatric patients, where long-term sequelae of both disease and treatment must be carefully balanced.
Teriparatide, a recombinant PTH (1-34) analog, has been increasingly utilized in the management of hypoparathyroidism, particularly in cases where conventional therapy with calcium and active vitamin D analogs fails to achieve biochemical control [3]. While intermittent teriparatide administration mimics physiological PTH secretion, there are few reports on its long-term efficacy and safety profile in pediatric patients [4].
Here, we report a pediatric hypoparathyroidism due to a de-novo CaSR mutation managed with teriparatide for more than ten years. This case provides unique insights into the long-term efficacy and safety, and practical challenges of PTH replacement in pediatric hypoparathyroidism.
In a guideline published by the Second International Workshop on hypoparathyroidism, keeping serum calcium level at low-normal range and vitamin D level at adequate range is recommended [5]. However, there is limited report demonstrating the importance of native vitamin D and calcium supplementation while the patient is on teriparatide treatment. In this case, we demonstrate a drastic reduction in teriparatide dosage requirement after supplementing calcium and native vitamin D.
Our findings highlight the importance of combined treatment strategies by teriparatide, native vitamin D and calcium therapy.
Our patient is a boy born at maturity of 36 weeks and 5 days with birth weight 3.46 kg, by normal spontaneous delivery. His Apgar scores were 8 at 1 minute and 10 at 5 minutes. The antenatal checkup and postnatal newborn screening examination were unremarkable. His newborn metabolic screening with dry blood spot test and thyroid function screening were normal. He was discharged on day 2 of life and was re-admitted for physiological neonatal jaundice, which was treated with phototherapy from day 4 to 5.
On day 12 of life, he was re-admitted for recurrent jittery episodes of around 10 seconds each. History did not reveal any fever, injury, focal symptoms or drug intake. Physical examination was unremarkable and did not elicit any focal neurological signs. Laboratory evaluation showed severe hypocalcemia, with serum ionized calcium 0.77mmol/L (reference range 1.15-1.35) & albumin-adjusted calcium (adjusted Ca) 1.82mmol/L (2.13-2.75), hyperphosphatemia at 4.09mmol/L (1.81-3.39), hypomagnesemia level 0.67mmol/L (0.82-1.62). Parathyroid hormone level (PTH) was suppressed to 1.3 pmol/L (1.6-6.9). Total 25-hydroxyvitamin D level was 37 nmol/L, suggestive of vitamin D insufficiency by Global Consensus [6] or vitamin D deficiency by Endocrine Society [7]. Paired spot urinary calcium-to-creatinine ratio was 0.94, which was inappropriately high while he was in severe hypocalcemia. Other extensive investigations, such as brain imaging, sepsis workup and metabolic screening were unremarkable.
With the coexistence of severe hypocalcemia with hypercalciuria and hyperphosphatemia, primary hypoparathyroidism was suspected. The diagnosis was established with genetic analysis after informed consent from parents. Targeted next-generation sequencing of the calcium-sensor panel genes was performed on peripheral blood, showing a heterozygous mutation of c.392G>A, p. (Cys131Tyr) in CaSR gene. This was a missense mutation from guanine to adenine in the nucleotide 392 of CaSR gene, leading to replacement of cysteine by tyrosine at position 131 in the CaSR protein, resulting in gain-of-function of the CaSR. It had been reported that cysteine residue at position 131 of the CASR protein is highly conserved across evolutionary distant species and CaSR c.392G>A, p.(Cys131Tyr) has been reported previously in hypocalcemic patients [8]. According to American College of Medical Genetics & Genomics joint consensus in 2015 [9], the mutation was classified as pathological. Parental analysis did not detect the CaSR mutation in either parent, whose plasma calcium concentration and urinary calcium excretion were normal, indicating the mutation had arisen de novo.
During infancy, his calcium and phosphate levels were fluctuating and frequent titration with calcium supplement up to 6mmol/kg/day was required to keep adjusted Ca level at low limit of normal. Alfacalcidol was added and gradually titrated up to 1.2 microgram daily. Nephrocalcinosis was first detected at bilateral medulla by surveillance ultrasound kidney at 11 months of age. Hydrochlorothiazide was added with potassium supplement at 13 months. Considering the renal complication and difficult-to-control calcium level and after detailed discussion of potential benefits and risks to parents, teriparatide (Forteo, Eli Lilly and Company, Indianapolis, IN), a recombinant, 34-amino-acid-sequence analog of human parathyroid hormone, was initiated at 2.5 microgram BD (0.5 microgram/kg/day) by subcutaneous injection at 16 months of age. Hydrochlorothiazide and potassium supplement were taken off after commencing teriparatide.
After the normalization and stabilization of adjusted Ca level, and in view of the co-existing renal complication of nephrocalcinosis, calcium and alfacalcidol were also taken off at 17 months of age. Throughout his childhood, the dosage of teriparatide was titrated up with weight gain and with the aim to achieve low-normal range of serum calcium. The dosage of teriparatide reached 37.5 microgram BD (2.85 microgram/kg/day), in order to achieve an adjusted Ca 2.1mmol/L (2.29-2.63) when he was 8.6 years old. Clarification with parents confirmed good adherence to treatment.
The serum 25-OH-vitamin D level was checked, and it was 40nmol/L, suggestive of vitamin D insufficiency by Global Consensus [6] or vitamin D deficiency by Endocrine Society [7]. Dietary history revealed he had limited sunshine and inadequate intake of dairy products and other calcium-rich food. Therefore, Calcichew D3 tablet daily (native vitamin D 800 units daily and calcium supplement 1000mg daily) were started at 8.6 years old.
Subsequently, after native vitamin D supplement, his teriparatide requirement was gradually decreased from 37.5 microgram BD (2.85 microgram/kg/day) at 8.7 years old to 5 microgram BD (0.35 microgram/kg/day) at 9.5 years old, to keep targeted adjusted Ca level (Figure 1).
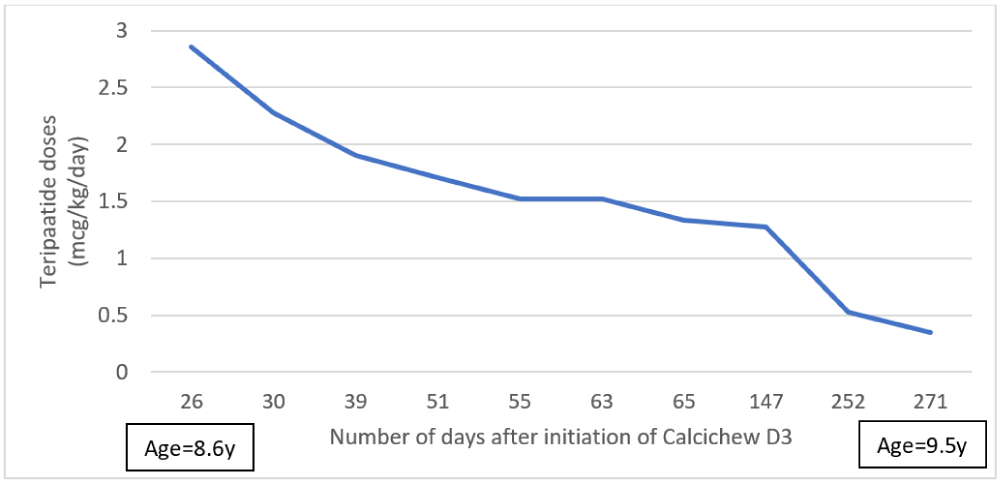 Figure 1: The trend of teriparatide dose after initiation of Calcichew D3 in days.
View Figure 1
Figure 1: The trend of teriparatide dose after initiation of Calcichew D3 in days.
View Figure 1
Observing the drastic reduction in teriparatide treatment requirement, calcium profiling over the 2 day was performed in him at 9.5 years old (Figure 2), to confirm the adequacy in treatment.
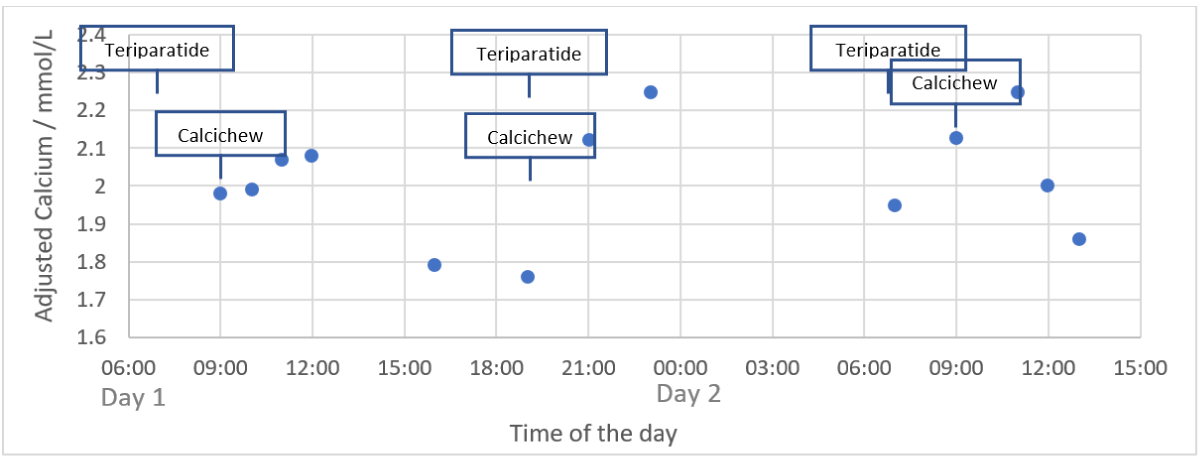 Figure 2: Serum calcium profiling in different timing of the day. Drug administration: (1) Teriparatide 5microgram SC at 7am & 7pm every day; (2) Calcichew D3 0.5 tablet (equivalent to 500mg elemental Ca + 400IU native vitamin D3) at 9am & 7pm every day.
View Figure 2
Figure 2: Serum calcium profiling in different timing of the day. Drug administration: (1) Teriparatide 5microgram SC at 7am & 7pm every day; (2) Calcichew D3 0.5 tablet (equivalent to 500mg elemental Ca + 400IU native vitamin D3) at 9am & 7pm every day.
View Figure 2
Observing the pre-dose hypocalcemia with adjusted Ca dropped to 1.76mmol/L at 7pm, the dose of teriparatide was increased to 7.5 microgram BD (0.52 microgram/kg/day), yielded stabilized adjusted calcium at around 2.3-2.5mmol/L post-dose during our follow-ups.
Currently, he is around 11.5 years old, and he has been on teriparatide treatment for more than 10 years. His latest 25-OH-vitamin D level was at 66nmol/L at age of 11.1 years. After the teriparatide, no clinical symptoms of hypocalcemia nor any adverse effect of treatment was shown. He was managed conservatively for his nephrocalcinosis, which remained asymptomatic and static in latest follow-up ultrasound kidney at 10.8 years old. Surveillance bone densitometry at 6.6 and 9.8 years old showed normal bone density. He enjoys normal growth and development, and he did not complain of any bone symptoms.
The CaSR is one of the heptahelical G protein–coupled receptors and is prominently expressed in the parathyroid glands and kidneys. The CaSR gene on chromosome 3p13, plays a critical role in calcium homeostasis by regulating PTH secretion. Gain-of-function mutations in CaSR lead to increased receptor sensitivity to extracellular calcium, lowering the serum calcium threshold for PTH suppression and enhancing renal excretion of calcium and magnesium. As a result, affected individuals may develop hypoparathyroidism and hypercalcinuria, and eventually present with hypocalcemia, hyperphosphatemia, and hypomagnesemia [10]. Our patient demonstrated good tolerance to teriparatide and without any reported adverse effects in long term.
This was compatible with other reports, which were very limited in pediatric population. Winer, et al. [11] conducted a 10-year longitudinal study on teriparatide use in 14 pediatric patients with hypoparathyroidism, observing normal bone accrual, mean serum and 24-hour urine calcium levels, and consistent calcium-phosphate product throughout therapy. Nephrocalcinosis was progressed in five patients who had pre-existing renal calcifications at baseline, though creatinine clearance remained unchanged. Reported side effects included bone pain in one patient and headaches in four individuals receiving teriparatide therapy. In adults, Marcucci, et al. [12] investigated teriparatide in patients with chronic hypoparathyroidism inadequately controlled by standard therapy. They found that a dose of 20mcg twice daily allowed most patients to discontinue calcium and calcitriol supplements while maintaining normal calcium, though some experienced fluctuations in calcium and phosphate levels. A larger scale phase 3 trial on another recombinant human parathyroid hormone (1-84) analog, on adult patients, the REPLACE trial [13] involving 134 cases, found that daily dose allowed over half of hypoparathyroidism patients to reduce their calcium and active vitamin D supplements while maintaining normal blood calcium levels. This study supported the treatment was generally well tolerated, with a safety profile similar to placebo. These limited reports advocate the use of recombinant PTH analogs as effective alternatives to conventional therapy for hypoparathyroidism.
In this case, we demonstrated the synergistic effect of combined treatment of teriparatide, calcium and native vitamin D. The commencement of teriparatide normalized his adjusted Ca level and prevented the progression of nephrocalcinosis. The supplement of calcium and native vitamin D significantly reduced his dosage requirement on teriparatide from 2.85 microgram/kg/day to 0.52 microgram/kg/day. The International Workshop on hypoparathyroidism guideline [5] suggested the importance of adequate vitamin D in patients. However, there is no consensus on native vitamin D supplement dosage while on recombinant PTH analog, and there are scarce clinical studies investigating the synergistic effect of native vitamin D and recombinant PTH. Nonetheless, our case demonstrated the role of adequate vitamin D. In our case, history revealed he had limited sunshine and inadequate intake of dairy products and other calcium-rich food, and thus 25-OH-vitamin D level was checked and confirmed vitamin D deficiency or insufficiency [6,7]. Therefore, low dose native vitamin D and calcium supplement was started to him and yielded drastic reduction on teriparatide dose.
One of the actions of PTH in calcium homeostasis is by regulating renal conversion of 25-(OH)-vitamin D to its active form 1,25-(OH)-vitamin D. PTH or its analogs stimulate the activity of 1-alpha-hydroxylase in kidney [14,15]. From our case, the dosage requirement of teriparatide was significantly reduced after the supplement of native vitamin D and calcium. It is pustulated that the slight insufficiency of 25-(OH)-vitamin D may affect the efficacy of teriparatide, as inadequate substrate in the conversion, even with activation of enzyme production. Also, vitamin D exerts in role in calcium homeostasis by enhancing intestinal calcium absorption. 1,25-(OH)-vitamin D regulates the active intestinal trans cellular pathway, via transient receptor potential vanilloid type 6 (TRPV6) channels for calcium trans membrane transport, calcium binding protein calbindin-D9k for intracellular calcium transport, and Calcium pumps for basolateral extrusion. Passive paracellular intestinal absorption via tight junctions is also influenced by vitamin D [16].
There are some in-vitro and animal studies demonstrate the synergistic effect of vitamin D on PTH actions. In a study concerning the response to infection in human keratinocytes and mice, 1,25-(OH)-vitamin D induced PTH receptor type 1 (PTH1R) expression, thus enabling response to PTH / PTH-related peptide and modulating immune response [17]. Therefore, there is a strong need for further evaluate on the synergistic effect of PTH action with vitamin D on calcium homeostasis and the clinical practice of combined treatment of recombinant PTH and native vitamin D.
In the management of our patient, serum calcium levels were monitored via intermittent blood sampling; however, this approach may not adequately capture diurnal variation influenced by therapeutic regimens, leading to challenge in drug titration. We decided to perform a calcium profiling over a 30-hour period to provide a dynamic assessment of serum calcium variations, revealing mild pre-dose hypocalcemia despite overall therapeutic stability with teriparatide. This highlights the limitations of isolated single calcium measurements and suggests that standardized calcium profiling could be a valuable tool for optimizing individualized treatment strategies in hypoparathyroidism.
This case represents one of the longest experiences in pediatric use of teriparatide for congenital hypoparathyroidism due to a de novo CaSR mutation, demonstrating its long-term efficacy and safety, providing our patient with sustained control without significant adverse effects and deterioration in complications including nephrocalcinosis.
The significant reduction of teriparatide dose after combining with calcium and native vitamin D underscores their synergistic roles in hypoparathyroidism. However, there is limited recommendations and studies on the combination treatment on hypoparathyroidism. Further studies are warranted to define optimal dosing protocols in combined therapy, evaluate extended PTH analog use in pediatric patients, and explore the role of real-time biochemical monitoring of serum calcium level in refining treatment strategies for hypoparathyroidism.
Both authors were involved equally in the diagnosis and management of this patient and manuscript submission.
No public or commercial funding.
This case report “Adequate 25-OH-Vitamin D Level and Calcium Intake Enhance the Efficacy of Teriparatide in Gain-of-function Mutation in Calcium-sensing Receptor Defect” is presented without conflicts of interest. Patient consent has been secured, and efforts made to anonymize identity. Findings are based on a single case, and the report does not endorse specific treatments. The authors encourage further research on this topic. Information provided is accurate to the best of authors knowledge.
Signed informed consent obtained directly from the father.