Stuttering is a speech disorder; the primary symptom in adults who stutter (AWS) is blocks, which halt both speech and breathing. This study aimed to evaluate vocal fold motion during blocks in AWS, in order to better understand this condition.
We used data obtained through flexible fiberoptic endoscopy and measurements of airflow and voice obtained from speech phonogram waveforms for 58 blocks in 12 AWS who were asked to read a set text for measurements. We compared the number of blocks with glottal closure and glottal opening during stuttering.
In most AWS, blocks were accompanied by both glottal closure and glottal opening. The proportions of blocks with glottal closure and glottal opening were 46.6% and 53.4%, respectively. Thus, vocal fold positions during stuttering blocks varied among individuals.
Our study shows that stuttering with cessation of voice can occur both when vocal fold is open and when it is closed.
Fiberoptic endoscope, Glottis closure, Stuttering, Vocal fold motion
Shortly after the onset of stuttering, preschool-aged children often show prolongation and repetition of initial syllables; these symptoms decrease with age. However, blocks that halt speech become more common with age [1,2]. Blocks occur unexpectedly, even for AWS themselves, and can also halt normal inspiration and expiration. This symptom resembles the chief complaints ("voice not coming out" and "clogged voice") made by patients with muscle tension dysphonia (MTD) and adductor spasmodic dysphonia (AdSD). A systematic review of voice therapy in MTD showed that there were positive changes to outcome measures immediately following a period of therapy [3], and that therapy for MTD continued to be effective for 6 months after the completion of therapy [4]. However, speech therapy for adults who stutter (AWS) is limited [5]. Stuttering is readily modified during treatment in the clinic, but this gain is difficult to transfer outside of the clinic; when it does transfer, the effect does not last long [6].
There have been many empirical studies, as well as theoretical descriptions, of the role of larynx during stuttering [7,8]. However, more objective information regarding the nature of laryngeal behavior during actual instances of stuttering is needed. The two general aspects of laryngeal activity for speech include phonation vibration [9] and abductory-adductory adjustment of the glottal aperture for voicing distinctions [10]. The latter adjustments are more likely to be related to the physiological disruptions associated with stuttering. MTD is a condition characterized by increased tension in the (para-) laryngeal muscles [11], often involving glottis closure. Very few studies have examined vocal fold motion in AWS. It has been reported that glottal closure occurred during 100% of blocks in a study of only six blocks [12]. However, repetition and prolongation during stuttering present both glottal closure and opening [12,13]. Since speech therapies for AWS are different from voice therapy in MTD, we suspected that vocal fold functions in stuttering differ from those of MTD and AdSD. Therefore, we hypothesized that stuttering blocks could also involve both glottal closure and opening. The present study aimed to evaluate vocal fold position during stuttering blocks using a larger dataset from AWS.
This study was approved by the Kyushu University Ethics Committee and conformed to the tenets of the Declaration of Helsinki. All patients provided written informed consent to participate in the study. The present study included 12 AWS (age range, 20-49 years; mean age, 31.2 years) and 10 adults with MTD (age range, 20-64 years; mean age, 35.3 years). In free conversation, it is sometimes difficult to discern whether stuttering has occurred, and people who stutter rephrase words to avoid stuttering. Therefore, in the present study, evaluations were conducted while the participants read a set text. To assess the frequency of stuttering, all participants were instructed to read 84 morae (seven sentences) from the book "Jack and the Beanstalk". Symptoms specific to stuttering (repetition of syllables, prolongations, and blocks) were recorded [14-16].
A flexible fiberoptic endoscope was inserted transnasally for the observation of vocal fold positions during the occurrence of blocks. Pitch, sound pressure, and expiratory flow rate measurements were obtained, and speech waveforms were recorded with an inserted mouthpiece, for all participants. Measurements were obtained using Lab Chart 8 Software (AD Instruments, Colorado Spring, CO, USA), followed by superimposition of laryngeal endoscopic images and phonogram signals (PS-77, Nagashima. Medical Instruments Co., Ltd., Tokyo, Japan). The analyses were conducted for measurements obtained during 58 blocks recorded from the 12 participants.
The stuttering blocks were classified on the basis of the vocal fold position as follows: Dystonic type, intermediate type, and lateral type, whose words were followed by vocal fold paralysis positions [17]. The dystonic type was represented by glottal closure, whereas the intermediate and lateral types were represented by glottal opening. Numbers of blocks with glottal closures and openings were recorded for all participants.
Table 1 shows the frequency of the dystonic (glottal closure), intermediate type (glottal opening), and lateral type (glottal opening) blocks in the 12 AWS. In most AWS, blocks were accompanied by both glottal closure and glottal opening. In three AWS (Cases 10, 11, and 12), blocks were accompanied only by glottal opening. Thus, there was no consistent trend. The proportions of blocks with glottal closure and opening were very similar, at 46.6% and 53.4%, respectively.
Table1 : Frequency of each type of block, classified according to the vocal fold position and the proportion of blocks with glottal closure and opening, in the 12 adults who stutter included in this study. View Table 1
Figure 1 shows the aerodynamic test results and speech waveforms during stuttering blocks. In the dystonic type, gradual cessation of the expiratory airflow was observed after the disappearance of speech waveforms, pitch, and sound pressure. In the intermediate type, there was no speech waveform, although slight respiration was observed. In the lateral type, there was no expired or inspired air and no phonation.
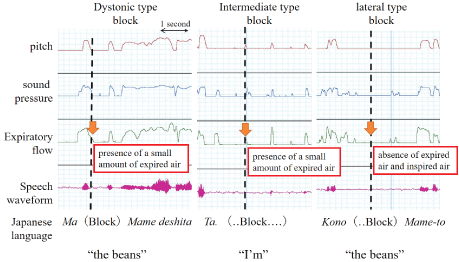 Figure 1: Representative cases of the three types of blocks classified in this study. The dotted lines indicate the timing of the occurrence of blocks.
View Figure 1
Figure 1: Representative cases of the three types of blocks classified in this study. The dotted lines indicate the timing of the occurrence of blocks.
View Figure 1
Figure 2 shows representative cases of MTD and expiratory flow rates. The expiratory flow of MTD decreased considerably; however, the waveforms for sound pressure and pitch were retained.
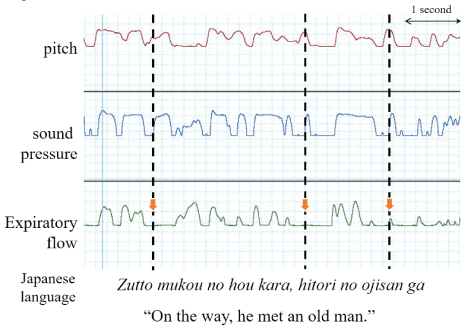 Figure 2: Representative cases of MTD.
Figure 2: Representative cases of MTD.
The expiratory flow has considerably decreased, but the waveforms for sound pressure and pitch are retained (dotted line).
View Figure 2
Figure 3 is a schematic diagram showing the differences among normal speech, MTD, and stuttering blocks. In normal speech, the respiratory centers in the pons and medulla oblongata are synchronized with the laryngeal motor neurons, and timing cues for the initiation are emitted from the speech centers in the cerebral hemispheres, resulting in vocal fold adduction, followed by the generation of speech. However, in stutter, there is a block in the timing cues for the initiation.
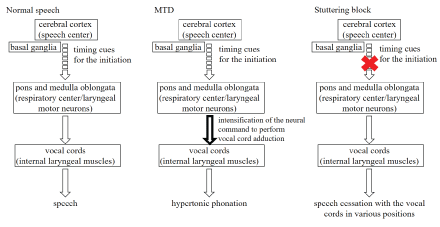 Figure 3: Differences among normal speech, MTD and stuttering blocks.
View Figure 3
Figure 3: Differences among normal speech, MTD and stuttering blocks.
View Figure 3
In the present study, we conducted measurements during 58 blocks in 12 AWS, and found 46.6% blocks with glottal closure and 53.4% blocks with glottal opening. Both glottal closure and glottal opening were observed in the same AWS, demonstrating that the vocal folds assume varied positions during stuttering blocks. This finding was not consistent with the findings of Conture, et al. [12], who examined only six blocks and found glottal closure in all of the examined blocks. Their study was limited by a small sample size, and the results should thus be interpreted with caution. We analyzed data related to 58 blocks in the present study and found an adequate number with glottal opening. In addition, vocal fold position was not constant during blocks in the same AWS.
In our study, pitch, sound pressure, and expiratory flow rate were measured simultaneously with speech waveform recordings. Our findings confirmed the presence of sound disruption during each stuttering block. During dystonic stuttering blocks, the disappearance of pitch, sound pressure, and speech waveforms were followed by a gradual decrease in the expiratory flow rate and its eventual disappearance. This phenomenon is characterized by a block accompanied by voice and was in contrast to the findings for the representative case of dystonic dysphonia described in Figure 2, which showed that expiratory flow was considerably lower in patients with MTD. The findings from the pitch and sound pressure waveforms suggest that voice disruptions occurring during dystonic stuttering blocks are not due to a blocked throat. In the lateral-type blocks, both voice and respiration were absent. In the intermediate-type blocks, voice disruptions occurred, although slight inspiration and expiration were present. The occurrence of blocks accompanied by voice can be attributed to limited respiration. In contrast to lateral blocks that are considered pure silent blocks, the intermediate blocks exhibited slight inspiration and expiration; these may be a combination of blocks and other types of stuttering. The above findings indicate that the position of the vocal folds is not constant during stuttering blocks and that both glottal opening and closure can accompany blocks in the same AWS. Therefore, treatment regimens, which are only useful to weaken vocal glottal closure similar to direct (specific laryngeal relaxation, Yawn-sing method, reduction of vocal loudness) and indirect therapy (managing the contributing and maintaining aspects of vocal abuse patterns or poor vocal hygiene) for MTD [3,4] and surgical treatment (type 2 thyroplasty) for AdSD [18], may not be effective in the treatment of stuttering.
Figure 3 shows the differences among normal speech, MTD, and stuttering blocks in AWS. In normal speech, the respiratory centers in the pons and medulla oblongata are synchronized with the laryngeal motor neurons, and timing cues for the initiation are emitted from the speech centers in the cerebral hemispheres, resulting in vocal fold adduction followed by the generation of speech [19-21]. MTD may be due to excessive neural commands responsible for vocal fold adduction, which are emitted by laryngeal motor neurons in the pons and medulla oblongata. However, blocks in AWS occur in various vocal fold positions, which suggests that the origin of this disorder is at a level higher than the respiratory centers in the pons and medulla oblongata, and that it does not involve the laryngeal motor neurons. The findings of this study are related to a deficit in brain timing networks of speech planning and timing cues for the initiation and execution of motor sequences in stuttering [22-24].
In conclusion, we found that stuttering blocks represent both glottal closure and opening, similar to prolongations and repetitions.
The authors thank Mr. Masakatsu Yamaguchi at Asahi Biomed for the tremendous efforts invested for obtaining the study measurements. We would like to thank Editage (www.editage.jp) for English language editing.
This work was supported by Grant-in-Aid for Young Scientists (B) 17K16922 (Y. K.) and Japan Agency for Medical Research and Development (AMED) (Y.K.).
The authors declare no conflict of interests.