Giant congenital melanocytic nevi (GCMN) are a rare occurrence. They arise from mutations in either BRAF or NRAS genes. The major complications associated with GCMN are malignant melanoma and neurocutaneous melanosis. Management of GCMN is symptomatic. We present a rare case of a 20-year-old woman born with GCMN.
Giant congenital melanocytic nevi, Neurocutaneous melanosis, Malignant melanoma
CMN: Congenital Melanocytic Nevi; GCMN: Giant Congenital Melanocytic Nevi; NCM: Neurocutaneous Melanosis; MRI: Magnetic Resonance Imaging
Congenital melanocytic nevi (CMN) are large brown-to-black skin lesions caused due to genetic mutations which leads to abnormal proliferation of embryonic melanoblasts [1,2]. Besides malignant transformation, patients with GCMN need to be periodically assessed for neurological abnormalities and psychosocial impairment. We present a rare case of a 20-year-old woman with GCMN, discussing its etiology and latest trends in management.
The patient is a 20-year-old woman who consulted for several pigmented patches over her body. The parents of the patient did not have a consanguineous marriage. None of the close family members had similar skin lesions. On examination, an extensive pigmented nevus was observed encompassing the neck, the back, the breast and the arms. Numerous smaller satellite nevi were also observed on the face, the gluteal region, the abdomen and the limbs (Figure 1, Figure 2 and Figure 3). No other congenital anomaly was observed and neurological examination was normal. A clinical diagnosis of GCMN was made. The patient was advised frequent follow-up visits and an MRI scan of the brain and spine was scheduled.
CMN arise from gain-of-function somatic mutations in either BRAF or NRAS leading to abnormal proliferation of embryonic melanoblasts [1-4]. CMN are predominantly caused by sporadic de novo mutations. However, some familial cases have also been reported [1,3].
CMN with a diameter greater than 20 cm have an estimated incidence of between 1/20,000 and 1/500,000 births [1,2].
CMN are characterized by their projected adult size, number of smaller satellite lesions, anatomical localization, and degree of rugosity, hypertrichosis, nodularity, and color heterogeneity [5]. A nevus with a projected adult size greater than 40 cm is classified as GCMN, as in our case. GCMN are further classified into six categories according to their anatomical location as bolero, back, bathing-trunk, breast/belly, body extremity, and body [5]. Our patient belonged to the bolero category.
GCMN are posed at a significantly greater risk of transforming into malignant melanomas, as compared to smaller-sized nevi (5 to 10%) [2-4,6]. Another complication associated with GCMN is neurocutaneous melanosis (NCM). It is characterized by abnormal melanosis of the central nervous system which is frequently asymptomatic at birth. It may manifest as hydrocephalus, lethargy, seizures, cranial nerve palsy, developmental delays, headache, and neuropsychiatric symptoms [2,3].
Management of GCMN is symptomatic and palliative. Surgical techniques include serial resection, excision followed by skin grafts/substitutes, and use of tissue expanders followed by resection [2,3]. Nonexcisional techniques for GCMN include dermabrasion, laser ablation, curettage, and chemical peel [3]. A novel surgical technique showing promising results is full-thickness excision of the nevus tissue, followed by its inactivation by high hydrostatic pressure (200 MPa for 10 min) and replanting it to its original site [6]. The use of an NRAS inhibitor (trametinib) is proposed for treating patients with an underlying NRAS mutation [1,2]. Use of endothelin-1 receptor antagonists has also been proposed. However, more clinical trials with larger samples size and longer follow-up periods are needed to determine the success rate of these techniques.
GCMN, although a rare condition, is associated with severe life-threatening complications such as malignant melanoma and NCM. Management includes surgical and non-surgical procedures, psychological intervention and clinical follow-up.
None.
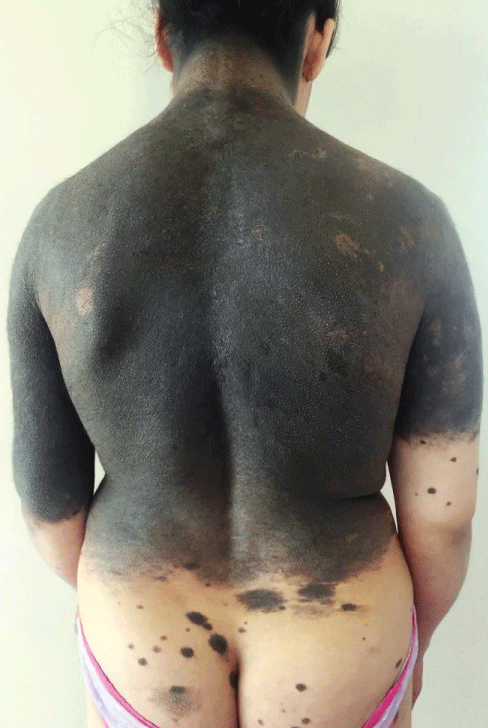
Figure 1: Giant melanocytic nevus encompassing the back and the neck. Numerous smaller satellite nevi are observed on the gluteal region.

Figure 2: Giant melanocytic nevus involving the breast with smaller satellite nevi on the abdomen.
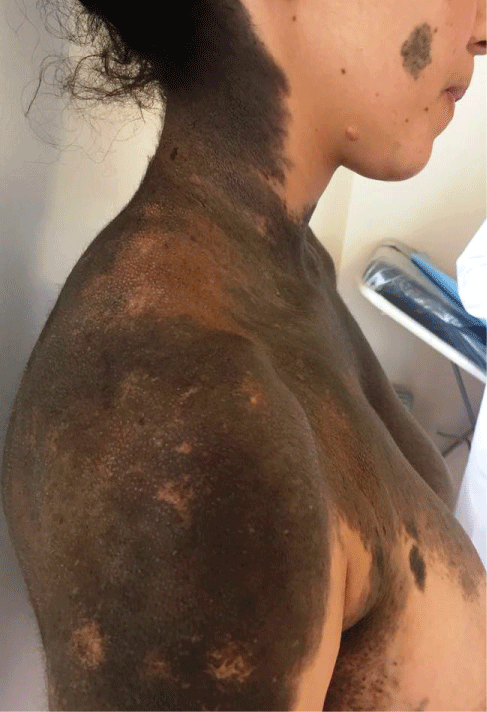
Figure 3: Extension of a giant melanocytic nevus to the neck with smaller satellite nevi on the face.