To define the serum pharmacokinetics of a fixed-dose of piperacillin/tazobactam at 24, 48, and 72 hours of therapy when administered as a continuous infusion in critically ill trauma patients.
Prospective, open-label pharmacokinetic analysis.
A Shock/Trauma Intensive Care Unit in a university hospital.
Ten adult patients with a documented Pseudomonas aeruginosa or Acinetobacter baumanii infection with a cefepime minimum inhibitory concentration (MIC) greater than or equal to 4 μg/mL.
All subjects received a 4/0.5 g bolus dose of piperacillin/tazobactam over 30 minutes followed by a 16/2 g per day continuous infusion. Serum piperacillin/tazobactam concentrations were analyzed at 30 minutes after the bolus dose (Cmax) and at steady-state (Css) during the continuous infusion (24, 48, and 72 hours). Piperacillin/tazobactam serum concentrations were determined using high-performance liquid chromatography.
The median piperacillin and tazobactam Css observed during the continuous infusion were 34.22 μg/mL (interquartile range [IQR], 28.91 - 48.21) and 5.49 μg/mL (IQR, 4.29 - 7.05), respectively. The median piperacillin and tazobactam Cmax after the initial loading dose were respectively, 72.28 µg/mL (IQR, 59.46 - 84.00) and 7.83 µg/mL (IQR, 6.94 - 9.09). A total of 96.4% (27/28) of the serum piperacillin Css measured were above the minimum inhibitory concentration (MIC) of the pathogen isolated, with 85% (24/28) being at least 2 times the MIC. For isolates with a MIC ≥ 32 μg/mL, only 53.6% (15/28) of the serum piperacillin Css were above the MIC.
Although we found a wide variability in serum piperacillin/tazobactam concentrations, the administration of a 16/2 g per day dose as a continuous infusion achieves optimal pharmacokinetic parameters for commonly isolated microorganisms in critically ill trauma patients. In the presence of elevated MICs (> 16 µg/mL) more aggressive dosing of piperacillin/tazobactam or use of an alternative antimicrobial may be warranted in this patient population.
Piperacillin, Tazobactam, Continuously infused, Critical care, Trauma, Pharmacokinetics, Pharmacodynamics
Optimizing the administration technique of β-lactam antibiotics has taken on a new horizon. For several decades, the standard practice has been by intermittent infusions. Although acceptable for most medications, intermittent infusions of β-lactam antibiotics can be debated. Recent pharmacokinetic studies demonstrate that intermittent administration of β-lactam antibiotics achieve insufficient drug concentrations in critically ill patients to provide maximal killing [1,2]. This practice occurs in intensive care units by clinicians extrapolating dosing recommendations from pharmacokinetic data in normal healthy adults and applying these data to critically ill patients. It is known that critically ill patients possess unpredictable pharmacokinetic parameters. The various characteristic changes seen in these patients are abnormal fluid status, hypermetabolic states and altered drug clearances [3]. These changes provide the clinician with a difficult pharmacokinetic environment.
While the pharmacokinetic profile determines the dosing interval and concentration in the serum or tissue, the clinical efficacy of these agents rely on the pharmacodynamic component. Pharmacodynamics involves the relationship between drug concentration and antimicrobial effect [4]. Time-dependent antibiotics, such as β-lactams, are bactericidal when their concentrations remain above the minimum inhibitory concentration (MIC) of the bacteria. Optimal killing with β-lactam antibiotics occurs when the serum concentration reaches four times the MIC of the pathogen [5] in combination with maintaining the trough concentration above the MIC90 for at least 50% of the dosing interval [6,7].
Combining pharmacokinetic and pharmacodynamic parameters of β-lactam antibiotics can be very challenging in the critically ill patient. An option to increase the dose results in increase cost of therapy and possibly exposing the patient to adverse drug reactions while shortening the dosing interval also has implications on resource utilization and cost.
A perfect medium for β-lactam antibiotics would be to find a dose that achieves adequate serum and tissue concentrations and maintains that concentration throughout the dosing interval. Using this method of drug administration would theoretically improve the efficacy of β-lactam antibiotics and reduce the potential for the development of bacterial resistance. Although undesirable, bacterial resistance to β-lactam antibiotics is becoming more frequent and is exacerbated by providing insufficient concentrations at the site of the infection. An option for maximizing the pharmacokinetic and pharmacodynamic profile of β-lactam antibiotics is to administer as a continuous infusion. Studies report continuous infusions of β-lactam antibiotics can achieve ideal pharmacokinetic and pharmacodynamic parameters in subjects with Gram negative infections at a smaller daily dose than intermittent administration [8].
Piperacillin/tazobactam is a broad spectrum, β-lactam antibiotic used for the treatment of a wide variety of infections caused by nosocomial pathogens. Piperacillin/tazobactam is commonly administered via intermittent dosing with the pharmacokinetics and pharmacodynamics being well established in normal healthy volunteers [9-11]. A recent study revealed inadequate pharmacokinetics and pharmacodynamics of piperacillin/tazobactam against Pseudomonas aeruginosa by intermittent infusion [11]. In light of the fact that critically ill patients display altered pharmacokinetics and are susceptible to life-threatening pathogens, it would be reasonable to alter the administration process of piperacillin/tazobactam.
In order to maximize the serum pharmacokinetic and pharmacodynamic effects of piperacillin/tazobactam, we evaluated a fixed dose of piperacillin/tazobactam as a continuous infusion in critically ill trauma patients with a documented Pseudomonas aeruginosa or Acinetobacter baumanii infection. The pharmacokinetics of piperacillin/tazobactam as a continuous infusion in critically ill patients has not currently been studied.
The primary purpose of this study was to define the serum pharmacokinetics of a continuous infusion of piperacillin/tazobactam in critically ill trauma patients at 24, 48 and 72 hours during the infusion. Secondary objectives were to report the maximum serum concentration of piperacillin and tazobactam after a single loading dose and to determine the pharmacodynamic parameters for the continuous infusion against Pseudomonas aeruginosa and Acinetobacter baumanii isolated.
The study protocol and informed consent procedure was approved by the Committee for the Protection of Human Subjects at Memorial Hermann Texas Medical Center prior to initiation. Informed consent from the patient or legal representative was obtained prior to enrollment in the study.
The study design was a prospective, open-label pharmacokinetic analysis. Initially the goal was to enroll 20 patients into the analysis but due to a nationwide shortage of piperacillin/tazobactam during enrollment, the study group size was reduced to 10. Inclusion criteria was 1) Patients admitted to the Shock/Trauma Intensive Care Unit, 2) Age greater than 18-years-old, 3) Creatinine clearance greater than 40 mL/min (estimated by using the method described by Cockcroft and Gault [12]), 4) Documented Pseudomonas aeruginosa or Acinetobacter baumanii infection with a cefepime MIC greater than or equal to 4 μg/mL, 5) And the decision of the critical care intensivist to start piperacillin/tazobactam as a continuous infusion. We choose a cefepime MIC of greater than or equal to 4 μg/mL because of the difficulty in achieving adequate pharmacodynamic parameters with cefepime by intermittent infusion at this MIC in this patient population. Therefore, it is the standard of practice in the Shock/Trauma Intensive Care Unit to begin a continuous infusion of piperacillin/tazobactam in patients infected with Pseudomonas aeruginosa or Acinetobacter baumanii that have a cefepime MIC greater than or equal to 4 μg/mL. Patients were excluded if they were greater than 130% of their admission weight or received piperacillin/tazobactam within 12 hours prior to enrollment.
Patients enrolled into the study received a single loading dose of 4 g/0.5 g piperacillin/tazobactam infused over 30 minutes. The drug was reconstituted per manufacturer instructions and diluted in 100 mL of 0.9% sodium chloride, USP. A blood sample (10 mL) for piperacillin/tazobactam concentration was collected 30 minutes after the end of the infusion of the loading dose. This blood sample represented the maximum serum concentration for piperacillin and tazobactam (Cmax). After administration of the loading dose and collection of the serum sample, 16 g/2 g piperacillin/tazobactam was administered over 24 hours as a continuous infusion each day for the duration of therapy. The continuous infusion was prepared in 250 ml of 0.9% sodium chloride, USP. This dose was selected because it was the same total daily dose as given by the standard regimen of 4.5 g every six hours which was given to patients in the Shock/Trauma Intensive Care Unit with documented Pseudomonas aeruginosa or Acinetobacter baumanii infections with a cefepime MIC ≥ 4 μg/mL. The infusion was administered at a rate of 10.4 mL/hour and was replaced every 24 hours when the daily dose was completed. Blood samples (10 mL) were collected at 24, 48, and 72 hours after initiation of the continuous infusion. These samples reflected steady-state concentrations (Css) of piperacillin and tazobactam. Blood samples were centrifuged, serum separated and stored at -70℃. Upon completion of the study, the serum samples were analyzed at an outlying laboratory for piperacillin and tazobactam concentrations via high-performance liquid chromatography (HPLC). A brief description of this method can be found in a previous citation [13]. An exact MIC of the organisms isolated was determined by E-test (AB Biodisk, Solna, Sweden) at Memorial Hermann Texas Medical Center microbiology department.
Demographic data collected for each individual patient enrolled was as follows: gender, age, temperature, drug allergies, admission weight, APACHE II score, serum creatinine, white blood cell count, percent bands, site of infection, organism isolated, exact MIC of organism, and concurrent antibiotics.
Pharmacokinetic parameters for continuous infusion piperacillin and tazobactam were calculated for each individual patient. Total body clearance (TBC) of piperacillin and tazobactam was calculated using the following equation,
where Ro is the infusion rate, and Css is the steady-state serum concentration measured. Area under the curve (AUC) was calculated using the trapezoidal method.
The following pharmacodynamic parameters were determined using the serum concentrations of piperacillin and tazobactam collected in the study: Cmax:MIC ratio at 24, 48 and 72 hours, area under the inhibitory curve (AUIC), and the percentage of serum concentrations above the MIC for both piperacillin and tazobactam.
Ten patients (eight males, two females) received the piperacillin/tazobactam continuous infusion for at least 72 hours with 40 blood samples collected for analysis. Two of the blood samples were excluded due to the concentrations being extremely elevated (2 piperacillin samples > 1000 μg/mL and 2 tazobactam samples > 350 μg/mL). The excluded samples were taken from patient #1 and patient #5 and were both drawn at the 72nd hour of the continuous infusion (See Table 1). Therefore, a total of 38 blood samples (10 following the loading dose and 28 during the infusion) were included in the analysis.
Table 1: Patient demographic data. View Table 1
The demographic data for the 10 patients is given in Table 1. The estimated creatinine clearance for all patients ranged from 83 to 129 mL/min at the start of the infusion, with the lowest and highest being 49 mL/min and 148 mL/min, respectively, during the infusion. One patient (#2) had the infusion discontinued because of the requirement of continuous veno-venous hemodialysis. Although the infusion was discontinued, the patient received at least 72 hours of the infusion and the data was included in the analysis. An APACHE IIInf score was not reported for patient #3 due to lack of laboratory data to complete the score at the time of infusion. Six patients received combination therapy with aminoglycosides during the continuous infusion.
Table 2 displays the serum concentrations and pharmacokinetic data for piperacillin. The peak serum piperacillin concentration (Cmax) extracted after a single loading dose did not have a considerable fluctuation. Overall mean Css for piperacillin observed was 50.26 ± 46.50 μg/mL during the continuous infusion. A sizeable standard deviation is noted in this value due to uncommonly elevated piperacillin concentrations observed from 1 patient (#2) included in the analysis. After removing this patient's Css from the analysis, the adjusted overall mean Css for piperacillin declines to 34.96 ± 12.36 μg/mL. The Css for piperacillin remained constant throughout the infusion period as illustrated in Figure 1.
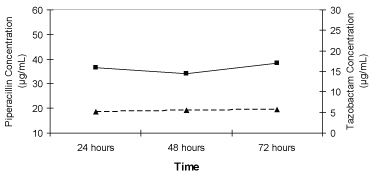 Figure 1: Median steady-state serum piperacillin (■) and tazobactam (▲) concentrations in critically ill trauma patients receiving 16 g/2 g piperacillin/tazobactam as a continuous infusion. View Figure 1
Figure 1: Median steady-state serum piperacillin (■) and tazobactam (▲) concentrations in critically ill trauma patients receiving 16 g/2 g piperacillin/tazobactam as a continuous infusion. View Figure 1
Table 2: Summary of piperacillin pharmacokinetics. View Table 2
The overall mean systemic clearance for piperacillin was 19.87 ± 11.07 liters/hour. We did observe a patient with a significantly lower than normal piperacillin clearance in which the mean clearance rises to 21.78 ± 10.11 liters/hour when eliminating this patient's data. AUC data for piperacillin is shown in Table 2.
The measured serum concentrations and pharmacokinetic data for tazobactam are reported in Table 3. As seen with the piperacillin data, tazobactam also displayed little deviation from the average maximum serum concentration. The overall Css for tazobactam during the continuous infusion was 6.76 ± 5.05 μg/mL. This value declined to 5.16 ± 1.58 μg/mL when the Css from a single patient (#2) were eliminated from the data set. On average, the tazobactam concentrations remained constant throughout the 72-hour infusion as seen in Figure 1. Overall systemic tazobactam clearance was 16.47 ± 8.1 liters/hour which increased to 17.94 ± 6.55 liters/hour without the data from patient #2.
Table 3: Summary of tazobactam pharmacokinetics. View Table 3
Table 4 shows the pathogens isolated along with the piperacillin and tazobactam MIC data. The percentage of Css for piperacillin that were above the MIC of the pathogen isolated was 96.4% (27/28). One piperacillin steady-state concentration observed during the infusion was below the MIC of the pathogen isolated. In this case, the Css of piperacillin measured was 11.54 μg/mL at 24 hours with the corresponding MIC of the pathogen being 16 μg/mL.
Table 4: Pathogens and minimum inhibitory concentration data. View Table 4
The mean Css/MIC ratio for piperacillin was 5.6 ± 4.3. Of the 28 Css for piperacillin observed, 11 were greater than 5 times the MIC of the pathogen isolated, which represented the most common group. A majority of the Css for piperacillin [14] were greater than 2 times the MIC with 13 concentrations occurring between 2 and 5 times the MIC. The percentage of Css for piperacillin above various MICs is shown in Table 5. The average AUIC for piperacillin was 112 ± 94.
Table 5: Percentage of steady-state serum piperacillin concentrations above the minimum inhibitory concentration for various minimum inhibitory concentrations at certain concentrations above the minimum inhibitory concentration. View Table 5
Of note, 5 steady-state tazobactam serum concentrations were below the MIC of the pathogens isolated.
Our study is the first pharmacokinetic and pharmacodynamic evaluation of piperacillin/tazobactam administered as a fixed-dose continuous infusion in critically ill trauma patients. Since 1997, there have been 10 published citations exploring the pharmacokinetic [14-19], pharmacodynamic [20,21], and/or pharmacoeconomic [22,23] effects of piperacillin/tazobactam via continuous infusion. Authors of these studies have incorporated population pharmacokinetics of piperacillin/tazobactam using data from normal healthy volunteers published in the literature and microbiology simulations to predict steady-state serum concentrations and pharmacodynamic parameters. The application of these data to critically ill patients, especially patients in the trauma unit, can be misleading.
In our current study, we utilized serum concentrations obtained from critically ill trauma patients at steady-state and applied these drug concentrations to determine the pharmacokinetic characteristics in this patient population. The results from our analysis displayed distinct differences from what is currently published in the literature profiling piperacillin/tazobactam as a continuous infusion. The mean steady-state serum concentration of piperacillin observed in our study was 50.26 ± 46.5 μg/mL. This mean steady-state serum concentration declined to 34.96 ± 12.36 μg/mL after excluding the data extracted from patient #2. We believe the latter serum concentration is more accurate due to the uncommon elevation of the serum piperacillin concentrations observed from patient #2 and the potential skewing of the data (wide standard deviations).
Serum piperacillin/tazobactam concentrations during a continuous infusion were first reported by Hitt and colleagues [14]. The investigators discovered that administering a daily infusion of 8 g/1 g of piperacillin/tazobactam to healthy adult volunteers, achieved an average steady-state serum concentration of 14.58 ± 5.19 and 2.35 ± 0.96 μg/mL for piperacillin and tazobactam, respectively. Recently, Chonghua, et al. [15] evaluated a daily continuous infusion of piperacillin/tazobactam (12 g/1.5 g) in patients with complicated intra-abdominal infections and reported a steady-state concentration for piperacillin of 35.31 ± 12.15 μg/mL. Using the same dose as in the previous study, Burgess and Waldrep [16] reported very similar steady-state piperacillin concentrations in eleven healthy subjects. Surprisingly, Buck, et al. [17] reported an average piperacillin concentration at steady-state of 39 μg/mL, administering 8 g of piperacillin continuously per day in hospitalized patients. There is one study available in which piperacillin serum concentrations were analyzed using a daily 16 g continuous infusion in eight patients with cystic fibrosis. Although the purpose of the study was to describe the nonlinear behavior of piperacillin using population pharmacokinetics, Vinks, et al. [18] observed a mean piperacillin concentration of 32.8 ± 14.4 mg/liter. Facca, et al. [19] also reported a steady-state serum piperacillin concentration of 53.4 ± 27.7 mg/liter using an unidentified dose. Although we observed very similar mean piperacillin steady-state concentrations, it should be noted that we administered 16 g of piperacillin continuously per day which is a 33-50% higher dose than the studies.
The difference in piperacillin concentrations observed from our study can possibly be explained by the type of patients enrolled. It is known that patients in the intensive care unit display extremely different pharmacokinetics than healthy subjects due to fluid shifts and altered drug clearances. Critically ill trauma patients, in particular, have the tendency to receive massive volumes of fluid for resuscitation and are commonly a young patient population. This is displayed in our data in which the average fluid balance at the time of the infusion was greater than 16 liters positive with an average patient age of 32 years. These two parameters play a major role when determining an appropriate dosing regimen for patients in this environment.
Since we obtained drug concentrations only at steady-state, we were unable to determine the volume of distribution for piperacillin/tazobactam. We were able to define drug clearance for both piperacillin and tazobactam. We found a mean drug clearance for piperacillin of 21.78 L/hr (excluding the data obtained from patient #2). This clearance is faster than what is reported in the literature. Healthy subjects have been reported to have a mean piperacillin clearance of 13.9 L/hr [16]. Other studies observed an actual or estimated clearance of 15.96, 8.9, 24.4, and 10.62 L/hr when piperacillin was administered as a continuous infusion [15-17,20]. The altered piperacillin clearance in our study is significant data in regard to the dosing of piperacillin in this patient population.
In addition, with most of the studies evaluating piperacillin/tazobactam as a continuous infusion focusing on the pharmacokinetics of piperacillin, there is very little pharmacokinetic data available on tazobactam administered continuously. This is the first pharmacokinetic analysis of tazobactam administered as a continuous infusion in critically ill trauma patients. We did observe consistent serum steady-state tazobactam concentrations throughout the analysis. The mean overall steady-state tazobactam concentration was 5.16 μg/mL (excluding data from patient #2). As with piperacillin, we found similar mean steady-state tazobactam concentrations from what is described in the literature, with our study using a higher daily dose (2 grams per day). In one study, Buck, et al. [17] reported a mean serum steady-state concentration of tazobactam of 6.3 μg/mL infusing 1 gram continuously per day. Another investigator used 1.5 grams of tazobactam infused continuously per day resulting in a mean steady-state tazobactam concentration of 7.29 μg/mL [15]. The results from another study revealed a serum steady-state tazobactam concentration of 2.3 μg/mL utilizing a 1.5-gram dose of tazobactam [16].
As seen with piperacillin, we found that the patients included in our study displayed a significantly faster tazobactam clearance than what is estimated and reported in the literature. The tazobactam clearance calculated in our study was 16.47 L/hr. In two studies evaluating tazobactam pharmacokinetics administered as a continuous infusion in hospitalized patients, the investigators reported a tazobactam clearance of 10.7 and 7.4 L/hr [15,17].
The significance of altered piperacillin/tazobactam clearance provides clinicians with the need to dose β-lactams more aggressively in this trauma patient population. For β-lactams, the pharmacodynamic parameter that correlates well with good outcomes is time above MIC (t > MIC). One way to maximize this parameter is to administer these agents as a continuous infusion. The results of our study indicate that piperacillin/tazobactam concentrations are maintained constant throughout 72 hours.
Despite the increased piperacillin clearance and potential volume issues, 96% of the piperacillin concentrations were above the MIC. Current data supports maximal killing effect occurring when the serum concentration for β-lactams is 2-4 times the MIC in conjunction with this concentration remaining above the MIC for at least 50% of the dosing interval. We found that 85% of the piperacillin steady-state concentrations were at least 2 × MIC of the pathogens isolated. The two organisms isolated in our study are commonly seen in critically ill patients. Also, both organisms were resistant to cefepime and a majority of the isolates had a piperacillin MIC of ≥ 16 (6/10). When using population pharmacokinetics of piperacillin/tazobactam, it would be expected that a dose of 16 g administered continuously per day would yield 100% of the serum piperacillin concentrations at least 2 × MIC for a MIC of 16. In light of these results, we can assume that extrapolating data from non-critically ill patients is not adequate to accomplish this.
The results of this study show administering 18 g daily as a continuous infusion of piperacillin/tazobactam to critically ill trauma patients achieve serum concentrations comparable to what is reported in the literature for non-trauma patients but at a higher dose. These findings provide clinicians with data showing increased systemic piperacillin/tazobactam clearance in critically ill trauma patients. The administration of piperacillin/tazobactam as a continuous infusion in critically ill patients, in general, may provide adequate serum concentrations especially in organisms with higher MICs. It is recommended that more aggressive piperacillin/tazobactam dosing be accomplished in the intensive care setting to maximize the effects of the drug. Moreover, the need for more studies evaluating the effects of piperacillin/tazobactam as a continuous infusion is warranted in the critically ill patient.
The pharmacokinetics of piperacillin/tazobactam is altered when administered to critically ill trauma patients as a continuous infusion. Despite the alterations in pharmacokinetic parameters, piperacillin/tazobactam displayed appropriate pharmacodynamic parameters in the pathogens isolated in the study using a daily 18 g dose. However, a dose greater than 18 g administered continuously may be needed in critically ill trauma patients when the MIC of pathogen is > 16 μg/mL. Further studies utilizing piperacillin/tazobactam as a continuous infusion are needed to evaluate the clinical efficacy of this method of administration in critically ill patients.
No financial interests to disclose.