REM sleep behavior disorder shares some clinical similarities with nocturnal epileptic seizures, which can result in misdiagnosis. We report a case of a man with Parkinson Disease diagnosis and parietal and frontal meningiomas who started to have abnormal sleep behaviors, suggestive of REM sleep behavior disorder. Video-polysomnographic showed periodic bilateral occipital epileptiform activity that occurred near arousals, where subtle movements were detected. During REM sleep no loss of atonia nor abnormal behaviors were detected. He was started on antiepileptic drugs, with improvement of the abnormal sleep behaviors. This case raises awareness for a possible role of epileptiform activity in patients with a REM sleep behavior disorder-like symptomatology.
REM sleep behavior disorder, Parkinson disease, Seizures
RBD: REM Sleep Behavior Disorder; PD: Parkinson's Disease; v-PSG: Video-Polysomnography; REM: Rapid Eye Movement; NREM: Non- Rapid Eye Movement
RBD is a parasomnia with a motor and behavioral pattern frequently associated with synucleinopathies. It can mimic focal epileptic seizures, mainly frontal and temporal lobe seizures [1,2]. These clinical similarities between RBD and nocturnal epileptic seizures may result in misdiagnosis [1]. The hyperkinetic motor manifestations reported in frontal epileptic seizures can be reminiscent of the brisk and violent arm and leg movements seen in RBD. Motor and verbal automatisms associated with temporal lobe seizures may mimic the gesturing and somniloquy observed in RBD [3].
Some studies indicate that epilepsy and RBD may coexist, but this information is very scarce [4,5].
Eighty-year-old man, with a PD for 8 years, PD-associated dementia for the last 3 years (Mini Mental State examination of 14, due to orientation, memory and attention errors), right frontal and left parietal meningiomas, treated testis neoplasm (2008), and nephrolithiasis, was observed due to abnormal sleep behaviors. These happened almost all nights, more than once per night, and were described as arm or leg movements, which sometimes were brisk like punching and kicking, and other times were like brief and low amplitude arm movements, with no apparent purpose. Straightforward stereotypy, dystonic posturing or automatisms were not reported by caregivers. The patient did not recall his movements. Sometimes he had vivid dreams with associated dream enactment. He also presented nightmares, daytime and nighttime visual and auditory hallucinations (description of people, associated with intense fear sensation), and occasional periods of persecutory delusions. These hallucinations were complex, not clearly stereotypical in content and they lasted long periods of time, unlike typical epileptic seizures. He never reported other daytime epileptic seizures. These features had started one year before, associated with excessive daytime sleepiness and snoring. At this time, he was on LDOPA/carbidopa/entacapone (300 mg levodopa)/day, rotigotine (4 mg/d), piribedil (100 mg/d), melperone (25 mg/d), and cyproterone acetate (100 mg/d). He stopped rotigotine and piribedil, and melperone was changed to quetiapine 200 mg/d, with resolution of hallucinations and delusions, but the abnormal sleep behaviors persisted. Given the previous history of meningiomas a video PSG with full 10-20 EEG montage was performed, in order to rule out epilepsy. On v-PSG, bursts of frequent brief periodic bilateral independent (right predominance) occipital epileptiform activity was detected, both in REM and NREM sleep (Figure 1). The patient had frequent arousals with evident subtle and varied, motor manifestations (brief myoclonus, discrete movements of arms and legs, swallowing movements), and occasional somniloquy with no understandable content and even shouting (Video 1). These arousals were preceded by periodic epileptiform activity that evolved to a generalized rhythmic delta activity frequently associated with persistent occipital periodic activity, a pattern that may suggest seizures, although it does not fulfill all neurophysiologic criteria. Definitive epileptic seizures were not registered in this examination. During V-PSG, 30 minutes of fragmented REM sleep was recorded. In this sleep phase there were frequent co-occurrence of phasic REM elements (rapid eye movements, myoclonus), and epileptiform activity. No abnormal behaviors or no signs of loss of atonia in tibialis anterior muscles were detected (the mentalis muscle had a persistent artifact that precluded its analysis). Moderated OSAS (AHI 19/h) was also diagnosed but the respiratory events were not associated with the aforementioned arousals that triggered subtle motor events. After the PSG, CPAP was started. There was no clinical improvement and the patient did not tolerate this treatment and maintained it for only 2 months, with insufficient compliance. Following v-PSG, given the possible presence of seizures during sleep, the presence of epileptogenic lesions epileptiform activity on the EEG, levetiracetam was introduced (500 mg, twice a day). Trazodone (150 mg/d) was also introduced. After this, abnormal sleep behaviors stopped. A routine EEG performed months later showed slow background with no epileptiform activity.
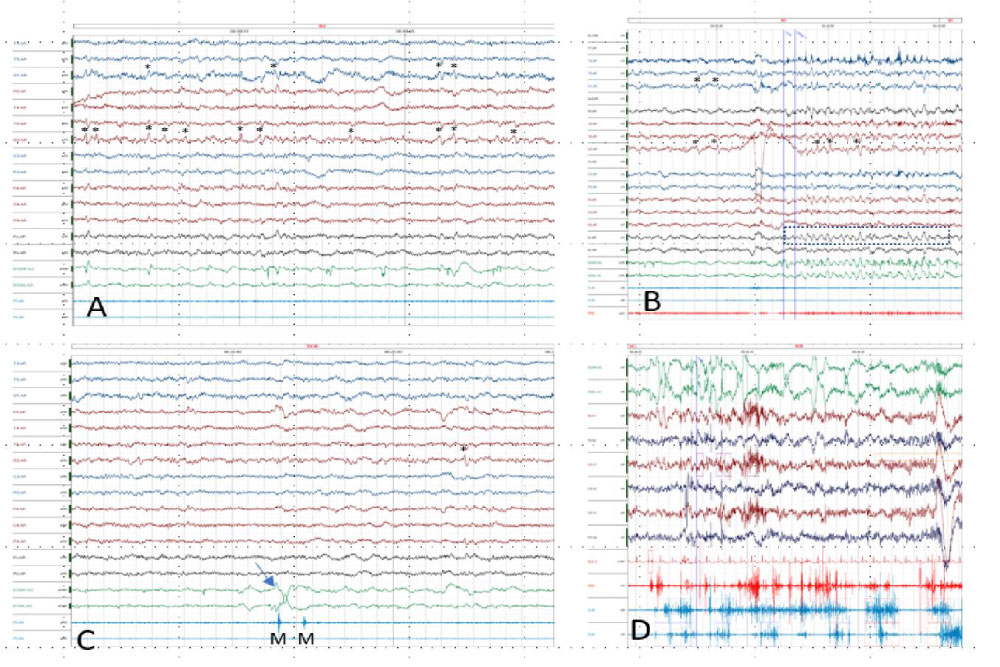 Figure 1: PSG images (A, B and C) showing bilateral occipital epileptiform discharges and typical RBD epoch from another patient for comparison (C).
Figure 1: PSG images (A, B and C) showing bilateral occipital epileptiform discharges and typical RBD epoch from another patient for comparison (C).
*: epileptiform discharges. The EEG electrodes are named according to 10-20 system. EOG: Electrooculogram; PLML and R - tibialis anterior surface EMG, left and right. EEG electrodes and FP1 and FP2 were not collected due to amplifiers constraints and F7 and F3 are absent due to artifacts. EEG filters: HFF 70 Hz; LFF 0.5 Hz; EMG filter: LFF 10, no HFF filter.
A: NREM sleep; B: NREM sleep arousal. Note the epileptiform discharges preceding the arousal followed by a rhythmic delta activity, mostly projecting in frontal lead (dotted box). The delta activity is also visible in EOG leads but this is due to EEG fronto-polar activity. During the arousal, occipital epileptiform discharges persist immersed in the delta activity. During this arousal, the patient performed mastication movements; C: REM sleep epoch. Note the rapid eye movements (arrow) and concomitant myoclonus (M); D: Typical RBD epoch from another patient, showing rapid eye movements, muscle activation in the mentalis (EMG) and PLMS and muscle artifact in EEG leads. View Figure 1
We describe a patient with PD and meningiomas with abnormal sleep behaviors that occurred during NREM arousals associated with frequent epileptiform activity.
PD has traditionally been considered a motor system disorder, but it is now widely recognized to be a complex disorder with diverse clinical features that include sleep disturbances [6]. Sleep complaints in these patients include insomnia, daytime sleepiness with sleep attacks, restless legs syndrome and sleep enactment behaviors, like the ones reported by our patient [7]. Sleep enactment behaviors in PD and other synucleinopathies are more frequently caused by RBD. This disorder is characterized by dream-enacting behavior that usually manifests as fighting behaviors and nightmares and occurs during REM sleep, associated with the loss of the normal REM sleep related atonia. Recently, however, other sleep behaviors termed "Arousal Related Motor Behavioral Episodes (AMBES)" have been described in these group of patients, albeit at lower frequencies than RBD. They can manifest as typical NREM parasomnias resembling sleepwalking or confusional arousals [8] and also movements that occur during brief arousals both in REM and NREM sleep [9,10]. Epilepsy is one of the most important differential diagnoses in parasomnias. Despite the fact that it has to be excluded for RBD diagnosis according to current criteria [11] most studies so far have focused in differentiating epilepsy from NREM parasomnias [12]. Very few studies have looked at the association or differential diagnosis between RBD and epilepsy [4,5]. Clinically, especially in elderly patients with synucleinopathies, with non-stereotypical, abnormal sleep-related behaviors, sometimes associated with dream content, epilepsy is rarely suspected.
Previous studies have found that epileptiform activity may occur in patients with clinical and neurophysiological diagnosed RBD. In a small series of 30 RBD patients, interictal epileptiform activity was found in 26.5% of these patients [5]. In those patients, these changes were considered to be a non-specific phenomenon related to brain aging. The same authors also reported the possible association between epilepsy and RBD in a small series of 6 patients, where it was suggested that REM sleep dissociation induced by RBD would increase the likelihood of seizures [4]. These few reports suggest that epilepsy or epileptiform activity on the EEG may be more common than usually accounted for in patients with RBD but these findings are usually treated as not clinically relevant. Our patient suggests, however, that in selected patients, these epileptic phenomena may be contributing to the clinical picture. The patient's v-PSG showed periodic occipital epileptiform activity, without concomitant RBD criteria, suggesting a possible link between sleep enacting behaviors and interictal epileptiform activity. V-PSG registered frequent, periodic, interictal epileptiform activity, with frequent arousals with minor motor phenomena preceded and associated with interictal epileptiform discharges. This motor phenomena consisted in brief myoclonus and swallowing movements, and occasional somniloquy. These events can be classified as AMBES, occurring in nonREM sleep. These events have been shown to occur more frequently in patients with PD associated dementia, like our patient, and Lewy body dementia, than in idiopathic PD [10]. We could not record a seizure and no clear-cut clinical description of seizures was made by caregivers. Nonetheless, some of the clinical description made by the caregivers could be interpreted as hypermotor seizures. Furthermore, the EEG pattern recorded during the arousals showed a rhythmic delta activity, that may occur in arousal disorders [13] but may also represent an ictal pattern [14]. In rare patients with Lewy body dementia, periodic EEG patterns have been described, but they are continuous generalized periodic discharges similar to Creutzfeld Jakob disease periodic discharges [15]. and not bilateral independent and paroxystic discharges, occasionally periodic, like the one we recorded in our patient. These type of EEG patterns have a strong association with seizures [14]. Taken together, the clinical suspicion, the PSG findings, the presence of congruent epileptogenic lesions and the clinical response to the antiepileptic levetiracetam led us to diagnose epilepsy in this patient. It also led us to hypothesize that, in our patient, interictal epiletiform activity (IEA) was triggering the arousals and, thus, the abnormal behaviors. Our hypothesis is that the IEA was mostly working as a non-specific trigger for arousal, in a mechanism similar to the paroxysmal arousals with minor motor events of sleep-related hypermotor seizures [16] that would later manifest as abnormal behaviors and somniloquy. As previously suggested [4], the increase in epileptiform activity in phasic REM sleep could have been due to REM state instability due to the neurodegenerative process, even if full RBD was not diagnosed in our patient. In the only previous studies that analyzed the frequency of sleep enacting behaviors and AMBES, epileptic patients and patients with structural brain lesions were excluded and there is no data in the literature regarding the co-occurrence of these two disorders. It is also possible that the increased IEA seen during arousal is non-specific, as this sleep-wake transitions enhance epileptiform phenomena. However, the rhythmic delta activity that follows and the possible response to levetiracetam favor a more causal relationship. It is noteworthy that trazodone was also introduced and may have contributed to a more stable sleep, with less arousals and behavior. Nonetheless, the absence of IEA in the follow-up EEG reinforces the relationship between the clinical improvement and epilepsy treatment.
An alternative hypothesis is that levetiracetam improved RBD alone. RBD has been shown to improve with a number of drugs, including levetiracetam, as reported in a single previous case [17]. In our patient's PSG REM sleep was very sparse and fragmented and it is not possible to completely rule out a concomitant RBD diagnosis. However, despite frequent, RBD is not present in 85-50% of PD patients [18]. It is interesting to note that the patient reported in the literature that showed improvement in RBD like-behaviors with levetiracetam had only a clinical diagnosis of RBD, showed diffuse slowing and nonspecific sharp waves on routine EEG, and never performed v-PSG. Therefore, it is not possible to completely rule out an epilepsy diagnosis in that case or a mechanism similar to the one we are suggesting.
The main clinical implication of this case is to raise awareness for a possible role of epileptiform activity in patients with RBD-like symptomatology and the necessity of neurophysiologic evaluations even in patients where clinical reasoning would clearly suggest a REM parasomnia. Making a diagnosis of epilepsy is a life-changing event. Besides chronic medication changes, it brings other important implications to everyday life. In our country, for instance, patients cannot drive unless seizure free for one year. Furthermore, there are still important social stigma associated with this diagnosis. Even in our patient, where PD associated dementia had already caused most of these changes, the decision to add chronic anti-epileptic drugs, with possible side effects and interactions, to an already poly-medicated elderly, should never be an easy one. Full v-PSG, with full EEG montages whenever the clinical suspicion of epilepsy is high, should always be performed.
Authors has no financial disclosure or conflicts of interest.
Isabel Loução Amorim, Ana Rita Peralta.
Isabel Loução Amorim.
Isabel Loução Amorim, Linda Azevedo Kauppila, Mariana Reis Costa, Carla Bentes, Ana Rita Peralta.