To evaluate the association of self-reported race with peripheral artery disease (PAD) and modification of this association by paraoxonase gene (PON1, PON2 and PON3) single nucleotide polymorphisms (SNPs).
This cross-sectional study included 11,992 Black or White participants from the Atherosclerosis Risk in Communities (ARIC) cohort with PON genotyping. Ankle brachial index (ABI) was measured at baseline (1987-1989); PAD was defined as ABI < 0.90. Data also included demographic, health and behavioral information. Logistic regression was used to evaluate the association between race and PAD after adjustment for age, gender, BMI, education, smoking, high cholesterol, hypertension and diabetes. The associations between PON SNPs and PAD were also assessed.
Blacks comprised 24.6% of the ARIC cohort; overall, 4.0% of participants had PAD. After adjustment for age, gender and BMI, Blacks had 1.27 times greater odds of PAD than Whites (95% CI = 1.04, 1.57), but this association became non-significant after adjustment for smoking, education, cholesterol, hypertension and diabetes. None of the SNPs evaluated met significance after Bonferroni correction for multiple comparisons (p < 0.001).
No association between race and PAD was identified after adjusting for health and behavioral covariates, suggesting that modifiable risk factors are major determinants of PAD. Further studies are needed to confirm this observation.
Ankle-brachial index, ARIC, Paraoxonase, PAD, Peripheral artery disease, SNP, Single nucleotide polymorphism
It is estimated that 12-20% of persons older than 60 years of age, or 8.5 million people in the US have peripheral arterial disease (PAD) [1]. PAD is a predictor of future CVD events, is prevalent worldwide, and found equally in men and women [1]. PAD occurs most often in the lower extremities and is defined as > 50% arterial stenosis as indicated by an ankle-brachial index (ABI) value < 0.9 [2]. ABI is a low cost, office-based assessment that accurately diagnoses PAD [2]. Normal ABI values range from 0.9 to 1.4 [2,3].
Risk factors for PAD include older age, high cholesterol, hypertension, diabetes and smoking [1]. Prior studies of the association between race and PAD have been inconsistent [4-6] with some reporting higher rates of PAD in Blacks but others failing to find racial differences. For instance, in 2.343 participants from the San Diego Population study, Criqui, et al. found that Blacks had higher odds of PAD (OR = 2.30, p < 0.024) than Whites after adjusting for covariates [7]. In contrast, there were no significant differences between Blacks and Whites in odds of PAD diagnosis (OR = 1.89; 95% CI = 0.89, 3.99) in a cohort of 403 patients from Houston, TX [8].
Additionally, genetics may modify the association between race and PAD. The development of atherosclerotic vascular diseases such as PAD are caused by oxidative damage from lipids and lipoproteins; this process may be mitigated by paraoxonase (PON) antioxidant enzymes [9-11] by reducing oxidative damage to low-density lipoproteins [12]. A recent case control study reported that PON1 concentrations and activities were lower in 66 PAD patients as compared to 8 controls [13]. Another case control study of 37 older participants (mean age 69.9 ± 9 years) with PAD found that PON1 genotype and PON1 activity had direct relations to brachial flow-mediated vasodilation (p = 0.0004) [12]. To our knowledge, no previous study has examined the possibility that PON genes moderate the association between race and PAD.
Objectives: The purpose of this study was to examine the association of Black and White race with peripheral artery disease, and to evaluate the effect of paraoxonase single nucleotide polymorphisms (SNPs) on this association using data from the Atherosclerosis Risk in Communities (ARIC) [14] Study cohort.
This study was approved by the University of California San Diego Human Research Protections Program (#160359X) and data was collected through authorized access from dbGaP. The ARIC [14] study collected data at four sites and was supported by the National Heart, Lung and Blood Institute (NHLBI) of the National Institutes of Health; each site obtained institutional review board approval and written informed consent from participants. All data analyses were performed using SAS® University Edition (SAS Institute, Cary, NC).
The Atherosclerosis Risk in Communities (ARIC) Study [14] is a prospective cohort study conducted in 4 communities in the United States (Washington County, MD; Forsyth County, NC; Jackson, MS; and Minneapolis, MN) each enrolling approximately 4,000 participants selected by probability sampling. A total of 15,972 study participants aged 45-64 years were examined at baseline (1987-1989) and re-examined every three years during four follow-up visits, with the last follow-up occurring in 2013. Since 2012, participants have been contacted annually by telephone to assess health status. Enrolled participants were Black (27%) and White (73%) men and women. Objectives were to investigate the etiology and natural history of atherosclerosis and to examine the risk factors and progression of subclinical to clinical cardiovascular disease events including coronary heart disease, heart failure, stroke and atrial fibrillation. In addition, the ARIC study examined genetic and environmental risk factors leading to ventricular dysfunction and vascular stiffness.
This study was limited to the 11,992 ARIC participants (24.6% Black, 75.4% White) with ABI values < 1.4 for whom PON genotyping data was available (Figure 1).
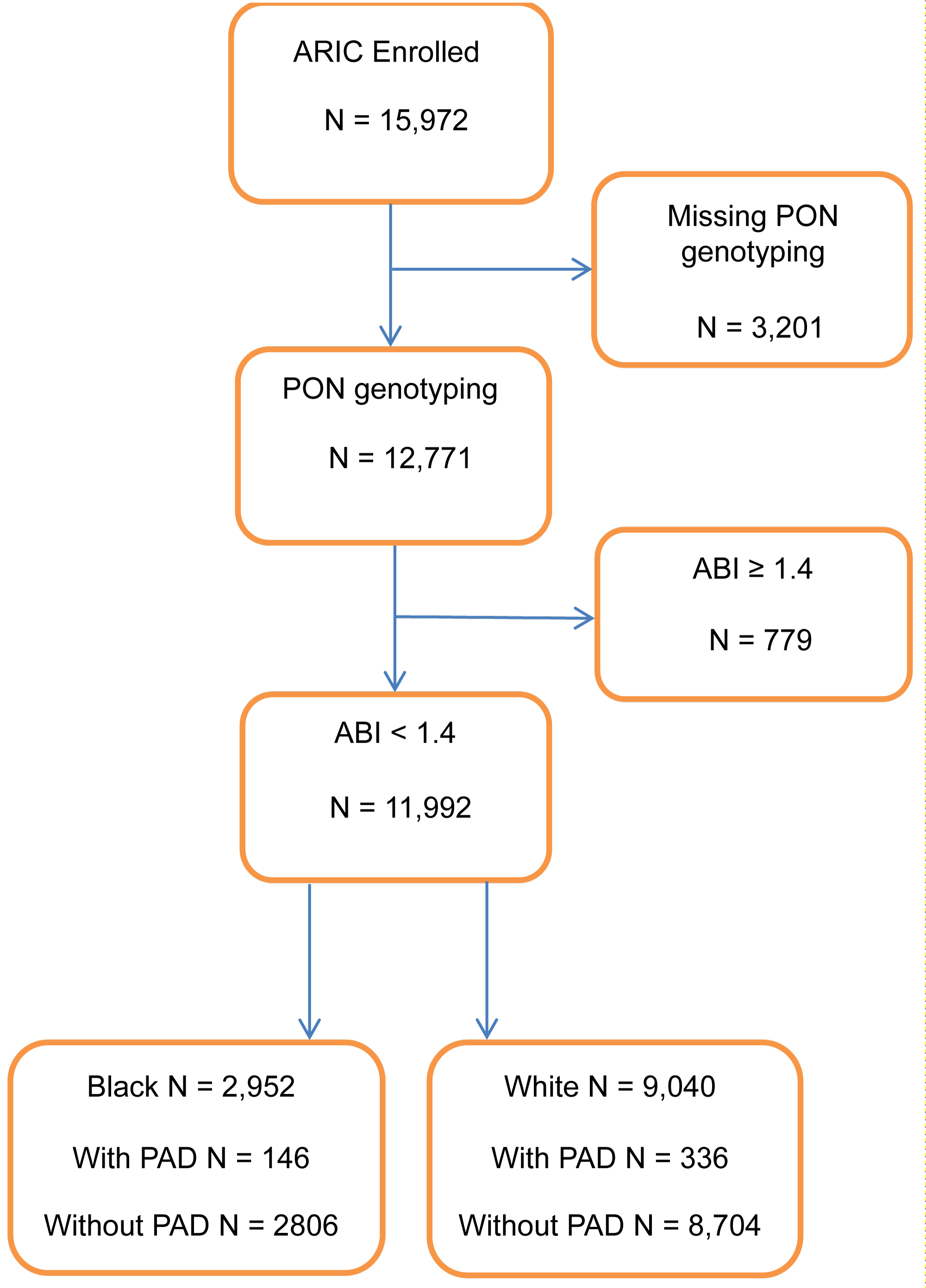 Figure 1: Sample population inclusion chart; ARIC, 1987-1989.
View Figure 1
Figure 1: Sample population inclusion chart; ARIC, 1987-1989.
View Figure 1
Race: In the ARIC [14] study, race was dichotomized as self-identified Black or White.
Peripheral artery disease: PAD was defined based on ABI values from the baseline visit. Single systolic blood pressure measures were taken in one upper extremity and one lower extremity to generate ABI values calculated as the ratio of lower to upper extremity [15]. Based on convention [1], participants with ABI values < 0.9 were categorized with PAD and participants with ABI values ≥ 0.9 and < 1.4 were categorized as normal and free of PAD. Participants with ABI values ≥ 1.4 (n = 779) were excluded from this analysis (Figure 1).
Covariates: Demographic characteristics (e.g., age, education, marital status), health history, body mass index (BMI) kg/m2 and results of fasting laboratory assays were obtained from the baseline visit [14]. Family history included maternal and paternal CHD events. Current marital status (yes/no), high school graduate or more education (yes/no), current cigarette smoking (yes/no) and alcohol use (yes/no) were assessed at baseline. Measures of systolic and diastolic blood pressure were obtained and 12-hour fasting glucose and cholesterol levels were assayed from blood samples at baseline. Participants taking anti-diabetic medication or having fasting glucose ≥ 126 mg/dl were categorized as having diabetes mellitus [16]. Those taking cholesterol-lowering medication or having laboratory assessed cholesterol > 240 mmol/L were categorized as having high cholesterol [17]. Participants taking antihypertensive medications or having systolic pressure > 140 mmHg or diastolic pressure > 90 mmHg were categorized as hypertensive [18]. Intake of current medications, including aspirin, anti-diabetic medication, antihypertensive medication and lipid lowering medication were determined by review of labelled containers [14] participants brought to the clinic visit.
Genotyping: Whole genome genotyping was performed using the Affymetrix 6.0 array platform [14]; there were 82 PON SNPs (43 PON1, 32 PON2, 7 PON3) available in the ARIC cohort which included (±) 20 kb window around each gene region. After excluding SNPs with minor allelic frequencies (MAF) < 5%, 62 SNPs remained available for screening analysis. All SNPs were in Hardy-Weinberg equilibrium and had ancestry-specific allele frequencies similar to those reported in publicly available databases (https://www.ncbi.nlm.nih.gov/projects/gapsolr/facets.html). The 62 SNPs were screened for significant association with PAD in Blacks and Whites combined and separately using a significance level of p < 0.001 (0.05/45) after Bonferroni correction for multiple comparisons confirmed 45 independent SNPs using the Nyholt method. Five principal component analysis covariates (PCs), obtained from the PLINK routine [19] were used to adjust for residual population stratification. SNPs were coded as continuous variables for analysis using an additive genetic model.
Statistical analysis: Descriptive statistics were calculated and reported as percentages for categorical variables and means (± standard deviations [SD]) for continuous data. Differences by race and PAD were analyzed using independent t-tests for continuous variables and chi-square analyses for categorical variables. All covariates were noncollinear based on correlation coefficients of r < 0.30. Covariates with at least marginally significant differences by race and presence of PAD as well as known confounders of the race-PAD association were retained for further analysis. Logistic regression was used to model the odds of PAD for Blacks compared to Whites. Statistical significance was defined as p < 0.05.
Forward stepwise multivariate logistic regression analysis was used to identify confounders (defined as a 10% change in the estimated coefficient between full and reduced models) and to assess the association between race and PAD, after adjustment for covariates. Model 1 examined the unadjusted association between race and PAD. Model 2 adjusted for age, gender and BMI. Model 3 adjusted for Model 2 variables and cigarette use. Model 4 adjusted for Model 3 variables and educational status. Model 5 adjusted for Model 4 variables and high cholesterol, hypertension and diabetes. None of the 62 PON SNPs screened met the significance criteria of p < 0.001) and were therefore not retained for further analysis. Interactions between race and covariates were evaluated with possible effect modification considered when p < 0.05.
Table 1 shows baseline differences for Blacks and Whites in the ARIC cohort. Peripheral artery disease as defined by ABI < 0.90, was found in 482 (4.0%) participants, with a higher prevalence in Blacks (5.0%) than Whites (3.7%) (p = 0.003). Blacks were younger (53.3 ± 5.8 vs. 54.3 ± 5.7 years respectively, p < 0.0001), but had a higher mean BMI (29.6 ± 6.0 vs. 26.9 ± 4.8 kg/m2, respectively, p < 0.0001) than Whites. There was a lower proportion of men (p < 0.0001) among Black participants and they were less likely to have a paternal (p < 0.0001) or maternal (p = 0.0002) family history of CVD than whites. Fewer Blacks used alcohol, completed a high school education, were married or used aspirin (p's < 0.0001). However, Blacks were more likely to smoke and to have a diagnosis of hypertension or diabetes (p's < 0.0001); there was no difference in prevalence of high cholesterol (p = 0.22).
Table 1: Baseline characteristics by race; ARIC, 1987-1989 (n = 11992). View Table 1
Comparisons of baseline characteristics by PAD diagnosis showed that participants with PAD were significantly older (55.4 + 5.9 vs. 54.0 + 5.7 years, respectively (p = < 0.0001) and had a higher average BMI 28.1 ± 5.9 vs. 27.6 ± 5.2 kg/m2 respectively, p = 0.018) (see Table 2). Those with PAD were more likely to be Black (p = 0.003), less likely to be male (p < 0.0001), and had a higher prevalence of smoking, hypertension, high cholesterol and diabetes (p's < 0.0001). However, those with PAD were less likely to be married (p = 0.0005), to have completed high school education (p < 0.0001) and to use alcohol (p = 0.018). None of the 62 PON SNPs in the ARIC population met the screening criterion for statistical significance (p < 0.001) and were therefore not retained for further analysis (Supplementary Table 1).
Supplementary Table 1: Association of SNP with peripheral artery disease (PAD) by race; Screening results of univariate logistic regression, ARIC, 1987-1989. View Supplementary Table 1
Table 2: Baseline characteristics by peripheral artery disease (PAD); ARIC, 1987-1989 (n = 11992). View Table 2
Step-wise logistic regression models (Table 3) showed that after adjusting for age, gender and BMI (Model 2), Blacks had 1.27 times greater odds of PAD than Whites (OR = 1.27, 95% CI = 1.04, 1.57). This association became non-significant in subsequent models that adjusted for cigarette use, educational status, high cholesterol, diabetes and hypertension (Models 3-5).
Table 3: Association of race with peripheral artery disease (PAD) after adjustment for PAD risk factors and SNPs; results of multivariate logistic regression, ARIC, 1987-1989. View Table 3
Main effects for all variables in Model 5 are shown in Table 4. Age, cigarette use, high cholesterol, hypertension (p's < 0.001) and diabetes (p < 0.01) were all significantly and independently associated with higher odds of PAD. Male gender was significantly associated with lower odds of PAD (p < 0.001). There were no significant interactions between race and any of the covariates.
Table 4: Adjusted independent associations of race, each covariate with PAD; results from multivariate logistic regression Model 5, ARIC, 1987-1989 (n = 11729). View Table 4
Atherosclerosis is the formation of fatty deposits of mostly low-density lipoprotein (LDL) cholesterol on the interior lining of the arterial walls [15]. Atherosclerosis manifests in a variety of ways, including peripheral artery disease (PAD) [4], and accounts for 1 in every 4 US deaths [20]. In this cross-sectional study of 11.992 ARIC cohort study participants, Blacks had higher odds of PAD (OR = 1.27, 95% CI = 1.04, 1.57) than Whites after adjustment for age, BMI, and gender, but this association was attenuated to non-significance after adjustment for comorbidities (i.e. hypertension and diabetes) and lifestyle risk factors (i.e. smoking, education). While this study did not find significant effect modification of PON SNPs on the association between race and PAD, to our knowledge this is the first study to report results of such an evaluation.
In this study of men and women aged 45 years and older from the ARIC cohort, there was a higher prevalence of PAD in Blacks than Whites (5.0% vs. 3.7%). These results are consistent with other [5,7], including Selvin, et al. who reported higher rates of PAD among Blacks than Whites (7.9% vs. 4.4%) using NHANES data [21]. Also, in accord with the NHANES data [21], we found a higher prevalence of cardiovascular disease risk factors including older age, higher BMI, current smoking, hypertension, high cholesterol and diabetes, among ARIC participants with PAD. However, our results suggested that race was not independently associated with PAD as adjustment for PAD risk factors such as smoking, hypertension and diabetes attenuated the association to non-significance. Results of the present study are in accord with Collins, et al., who also reported no association between race (Black or White) and PAD after adjustment for diabetes, smoking, hypertension and age [8].
In contrast, studies from selected populations have reported associations between race and PAD [5,7]. For example, the San Diego Population study [7] reported that Blacks had higher odds of PAD than Whites (OR = 2.30, p < 0.024). However, that study included participants with prior revascularization, which normalizes ABI, resulting in a higher prevalence of PAD than observed in ARIC. In addition, a study based on the MESA cohort reported that Blacks had significantly greater odds of PAD than Whites (OR = 1.67; 95% CI = 1.23, 2.26) after adjusting for multiple covariates [5]; however, MESA excluded those with known CVD, making a direct comparison with the ARIC cohort, which had no such exclusions, difficult.
The lack of a significant association between race and PAD after adjustment for health and behavioral CVD risk factors in the present study suggests that differences in PAD are influenced more by racial disparities in risk factors and their management rather than racial differences in the biological development of atherosclerosis. Prior research reports that Blacks present at a later clinical stage in the development of PAD than Whites. Furthermore, diabetes and neuropathy, both more prevalent in Blacks, affect the distal arteries and contribute to a PAD diagnosis [22]. Finally, it has been previously demonstrated that racial disparities exist in health care, with Blacks receiving lower quality than the majority of Whites in the United States [23-27].
The influence of genetic differences on the risk of PAD is relatively unknown. PON1 enzymes have anti-inflammatory and antioxidant properties and may protect against atherosclerosis [28]. The enzyme is expressed in the liver and delivered to multiple tissues not expressing the enzyme [28]. Decreased PON1 enzyme activity has been shown to increase inflammation in animal studies, and increase oxidative stress among patients with atherosclerosis, diabetes and/or hypercholesterolemia [28]. Recent studies report associations between PON SNPs and atherosclerotic disease or related phenotypes. The PON1 rs2299260 SNP was significantly associated with a 50% increased risk of resistant hypertension in European-Americans for each additional copy of the C allele [29]. Additionally, another PON1 promoter region SNP, rs705379, was associated with blood pressure in middle-aged individuals [30]. However, others have failed to find an association between PON1 polymorphisms and cardiovascular disease. In two separate meta-analyses, no association was found between a L55M PON1 polymorphism and ischemic stroke [31], and no association was found between the 55L allele and stroke after adjusting for risk factors [32].
It is biologically plausible that there is an association between race and PAD after adjustment for risk factors. Previous studies show that Blacks have a thicker and stiffer aorta compared to Whites [33] and aortic stiffening may artificially lower ABI measures. Aboyans, et al. found that among the healthy participants of the MESA cohort, Blacks had ABI values approximately 0.2 lower than Whites [22]. We may have failed to find this association in adjusted models because of the small sample of Blacks relative to Whites in this cohort. Future studies assessing the genetic differences and gene-environment interactions with respect to PAD need to be evaluated across diverse ethnic study populations with adequate sample sizes [34].
Several limitations and strengths of this study were considered. ABI may be underestimated due to categorization based on a single measure of systolic blood pressure from one upper and one lower extremity and rather than multiple measures in all extremities. The ARIC cohort may not be comparable to other cohorts such as MESA that have reported an association with race and PAD because of differences in selection criteria [35]. Misclassification due to self-identified race and ascertainment of other risk factors may contribute residual confounding affecting the estimation of the association between race and PAD. Here PAD was defined as ABI < 0.90 without consideration of prior revascularization, which would normalize ABI, potentially underestimating the prevalence of PAD [36]. Finally, this study excluded participants with an ABI ≥ 1.4 because of arterial stiffening due to calcification; it is estimated that PAD prevalence affects approximately 1% of persons in this group [7], resulting in an underestimation of the prevalence of PAD. This study also has several strengths including the use of data from a relatively large cohort of both Black and White men and women who were enrolled using a standardized protocol. This study also adjusted for educational status, which could contribute to differences in diagnosis, access to care and treatment. Finally, the effects of genetics as well as the interaction between race and PAD risk factors were examined.
While this study found an overall higher prevalence of PAD among Black participants, race was not significantly associated with PAD after adjusting for health and behavioral risk factors PAD. This suggests the risk of PAD may be modified through medical management and lifestyle changes. To our knowledge, this is the first study assessing the effect of the PON gene cluster (PON1, PON2 and PON2) on the association between race and peripheral artery disease. Additional studies are needed to further assess the impact of genetics on the association between race and peripheral artery disease.
This study was approved by the University of California San Diego Human Research Protections Program (#160359X).
No conflicts of interest.
All authors who significantly contributed to this manuscript have been listed.
No sources of funding.