Overweight and Obesity have been associated with an increased risk of developing a variety of gastrointestinal cancers [1]. The increasing implementation of bariatric surgery to manage overweight and obesity would likely increase the incidental diagnosis of malignancies in patients who were previously asymptomatic or had nonspecific gastrointestinal symptoms. We present the case of a MALT gastric lymphoma incidentally diagnosed on histopathology following laparoscopic sleeve resection for obesity.
Gastrectomy, Bariatric surgery, Obesity, Lymphoma, Gastrointestinal tumour
A 59-year-old female underwent standard laparoscopic gastric sleeve resection following failure of diet and medical management for obesity (weight, 121.4 kg; BMI, 45 kg/m2). Comorbidities included hypertension, hyper triglyceridaemia and insulin resistance. Routine preoperative workup including upper gastrointestinal ultrasound, and barium swallow were unremarkable.
Following routine 5 port optical entry, the greater curvature vessels were divided. The sleeve resection was performed using an Endo GIA Tristapler (Medtronic, Minneapolis). The staple line was reinforced with seamguard and imbricated proximally with 3.0 Prolene sutures. There were no intra-operative complications.
Macroscopic evaluation of the resected gastric specimen revealed an elevated mass measuring 180 × 30 mm at the posterior wall, involving the mucosa and muscularis propria of the fundus, and extending anteriorly by 60 × 25 mm (Figure 1). The fundal lesion consisted of a diffuse lymphoid infiltrate from the mucosa to the muscularis propria. Atypical small to intermediate cells were noted on microscopy with occasional lymphoepithelial lesions and residual reactive follicular centres. An area of necrosis within the tumour was also identified. Lymphoid immunoperoxidase staining demonstrated:
CD20 and bcl2: positive CD5, CD10, CD23, bcl6 and cyclin D1: negative Ki-67 proliferative index of 5% Helicobacter pylori (H. pylori) stain: negative.
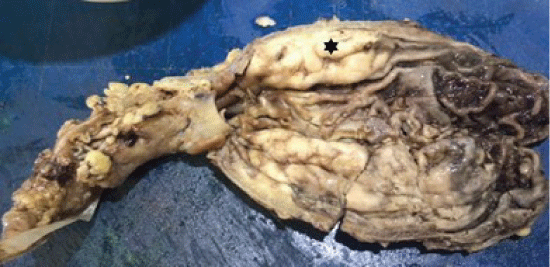 Figure 1: Macroscopic view of gastric fundus with MALT lymphoma (★). View Figure 1
Figure 1: Macroscopic view of gastric fundus with MALT lymphoma (★). View Figure 1
A diagnosis of extranodal marginal zone B cell lymphoma of MALT (Mucosa Associated-Lymphoid Tissue) type was confirmed.
Post-operatively, the patient was reviewed by a hematologist who noted her to have been asymptomatic prior to the operation but for a single episode of non-specific dysphagia and upper abdominal pain. Clinical examination revealed no lymphadenopathy or hepatosplenomegaly. Lymphoma staging with computed tomography did not show clear evidence of local spread or distal lymphadenopathy. Full blood panel was within normal limits. Peripheral blood smear did not show any evidence of lymphoproliferative disorders. Post-operative urea breath testing for H. pylori was negative. Following multidisciplinary evaluation, she was placed onto a twice yearly endoscopic surveillance program, of which the two most recent surveillance results demonstrated no lymphoma or H. pylori in all biopsies tested. At one year post-operatively, the patient had lost 31.0 kg (BMI, 33.89 kg/m2) with no post-operative complications.
Extranodal marginal zone B-cell lymphoma of MALT type are the most prevalent of the heterogeneous gastrointestinal lymphoma group, accounting for approximately 35% of primary gastric lymphomas [2].
Gastric MALT lymphoma almost exclusively affects adults. Clinical features are often nonspecific and include abdominal discomfort, vomiting and diarrhoea. Commonly, nonspecific upper gastrointestinal symptoms lead to incidental discovery of MALT lymphoma on endoscopic biopsy [3]. Management is dependent on sufficient biopsies being retrieved from the lesion for histopathological diagnosis and to concurrently rule out possibility of diffuse large B cell lymphomas. Once diagnosed, recommended staging investigations include esophagogastroduodenoscopy with multiple biopsies taken from each region of the stomach; endoscopic ultrasound to evaluate regional lymph nodes and gastric wall infiltration; bone marrow aspirate and biopsy in addition to routine biochemical and radiological investigations [4].
Gastric MALT lymphomas are overwhelmingly linked to concurrent gastroduodenal infection by H. pylori [5]. Evidence for the pathogenic relationship include the identification of H. pylori in the gastric mucosa of 70-90% of patients with MALT lymphoma, and in vitro studies which have demonstrated the stimulation of lymphoma growth in culture when exposed to H. pylori strain specific T cells [6,7]. MALT lymphoma in our H. pylori negative patient is therefore an unusual finding. Pathogenesis of H. pylori negative MALT lymphoma is unclear. Genetic alterations in nuclear factor-kappa activation, and infection by other bacteria have been implicated [8]. Where the presence of active H. pylori is not demonstrated by immunohistochemistry, further investigations including serology, urea breath test or stool antigen test must be performed to rule out infection [9].
The prevalence of H. pylori in obese patients compared to the general population has been variably reported with rates affected by geography and level of economic development. A meta-analysis of 18 studies failed to demonstrate an association between H. pylori infection and BMI [10]. In contrast, a recent an ecological review of 49 studies has demonstrated a significant inverse relationship between H. pylori colonisation and prevalence of overweight and obesity in the developed world [11], highlighting disagreement in evidence.
Routine surveillance of H. pylori is not a feature of pre-operative assessment in our bariatric practice. Indeed, a lack of consensus on routine pre-operative screening for H. pylori in all bariatric patients exists in the literature. The American Association of Clinical Endocrinologists/The Obesity Society/American Society for Metabolic and Bariatric Surgery guidelines recommend routine preoperative H. pylori screening only in high prevalence areas [12]. In the setting of Roux-en-Y gastric bypass, no difference in complications such as anastomotic ulcer rates between H. pylori tested and non-tested patients has been reported [13]. Despite this, pre-operative eradication of H. pylori in bariatric patients has been advocated by various authors for reasons including the potential for gastric ulceration and malignant transformation in the post-operative gastric remnant, which may be difficult to access postoperatively during endoscopic evaluation, and therefore progress undetected, and the non-invasive and validated cost-effective nature of the surveillance process [14].
A recent prospective study by Abdullgaffar and colleagues [15] calls into question the utility of routine microscopic examination of all laparoscopic sleeve gastrectomy specimen. Based on < 1% incidence of benign lesions and no malignant lesions identified in 546 specimens, they advocate special gross handling protocols and meticulous macroscopic evaluation with selective microscopic examination only, for the benefit of cost and time effectiveness whilst demonstrating no major compromise to patient safety. Further evidence on this position in gastrectomy procedures is however lacking. Our institutional practice is for thorough histopathological evaluation of all resected specimen in sleeve gastrectomy, and the above case highlights the potential for missing incidental pathologies, despite their rarity, leading to adverse patient outcomes.
In H. pylori positive MALT lymphoma, detected prior to, or following bariatric surgery, a combination proton pump inhibitor and clarithromycin based triple therapy has demonstrated a remarkable success rate with complete H. pylori eradication in localised gastric MALT lymphoma leading to tumour regression or complete cure in over 75% of patients [16]. Interestingly, several studies have also demonstrated success in H. pylori therapy for some H. pylori negative MALT lymphoma patients [17,18] implicating other aetiologies in the development of MALT lymphoma.
For patients with clearly defined minimal histological residual disease following complete H. pylori eradication, Fischbach and colleagues [19] demonstrated a 'watch and wait' strategy with regular endoscopic surveillance and biopsies as a valid approach rather than further escalation in management. Unsurprisingly, antibiotic resistance or disseminated disease require urgent consideration of oncologic treatments. Radiotherapy has shown to be slightly superior to chemotherapy for induction of remission in pooled data, particularly for stage IE-IIE disease. The role of surgical intervention is reserved for rare cases of MALT lymphoma associated complications such as perforation or bleeding not amenable to endoscopic control [9].
Importantly, MALT lymphoma patients have demonstrated increased incidence of gastric and non-Hodgkin lymphoma in addition to risk of recurrence [20]. In patients who have achieved cure, close follow up is therefore necessary with endoscopy and biopsy mapping the key surveillance modality recommended despite exact time interval and duration of follow-up being unclear.
Bariatric surgery is increasingly utilised in the management of overweight and obesity worldwide. Our case highlights the need for thorough pre-operative patient workup, and the utility of routine histopathological analysis of resected specimen in bariatric surgery for identification of uncommon pathologies such as MALT lymphoma.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The article has not been published in any other source. All content is original. There are no conflicts of interest to declare.