Brain radiation necrosis (BRN) is a side effect of radiotherapy (RT), affecting mainly the white matter and can appear from a few weeks to several years after RT. It's incidence of 3-9% is increasing as survival increases. Histopathology (HP) shows avascular damage, demyelination and direct necrosis.
Brain radiation radionecrosis and the tumor progression are difficult to differentiate as both entities are presented with similar radiological and clinical characteristics, such as neurological deficit, brain edema and contrast enhancement.
Recurrent aggressive BRN is infrequent. Big recurrences in a few months is possible but uncommon. BRN still remains an important diagnostic challenge to determine the best treatment option. This entity can present with important neurological deficits, big mass lesions and edema and may require multiple surgeries to control its growth and improve the clinical status. The 5-ALA intraoperatively, could help to assess the presence of tumor intraoperatively. In some cases, hyperbaric oxygen and anti-VEGF (vascular endotelial grow factor) therapy may be useful as treatments when surgery cannot be done or it's been refused.
Radionecrosis may behave more aggressively than high-grade glioma itself. Sometimes it is necessary to perform several interventions and therefore increase the morbidity and mortality in these patients.
Hyperbaric, Brain tumor, Radiation necrosis, Radiotherapy, 5-Aminolevulinic acid
HP: Histopathology; RN: Radionecrosis; RT: Radiotherapy; CTx: Chemotherapy
The Brain radiation necrosis (BRN) is a side effect of radiotherapy (RT), affecting mainly the white matter and can appears from a few weeks to several years after radiotherapeutic treatment has ended [1,2]. The BRN has an incidence of 3-9% and is increasing as survival increases. It's accepted that dose < 60 Gy in 2 Gy daily fractions are safe and with a lower incidence of BRN (5%), considering this as the usual protocol for high-grade gliomas (STUPP) [1-4]. The BRN histopathology (HP) shows: A vascular fibrinoid necrosis, endothelial damage, increased capillary permeability and cerebral edema. Also, direct damage of RT on glial cells, because of their high sensitivity to it, producing demyelination and direct necrosis. Some new theory argues that the effect of host immune response is through inflammatory cytokines [1-3,5,6].
Brain radiation necrosis and the tumor progression are difficult to differentiate as both are presented with similar radiological and clinical characteristics, such as neurological deficit, brain edema and contrast enhancement. The MRI perfusion/spectroscopy or PET-CT (FDG) have a high rate of false-negative-/false-positive. Surgery plays a therapeutic and diagnostic role (Gold standard = HP evaluation) [1-4,7].
48-years-old female, with partial seizures on the left side. The MRI shows a big right frontal lesion, solid-cystic 4.2 × 3.7 × 4.5 cm with contrast enhancement (Figure 1.1). Subtotal removal is performed (> 90%) and no deficits (Figure 1.1.1).
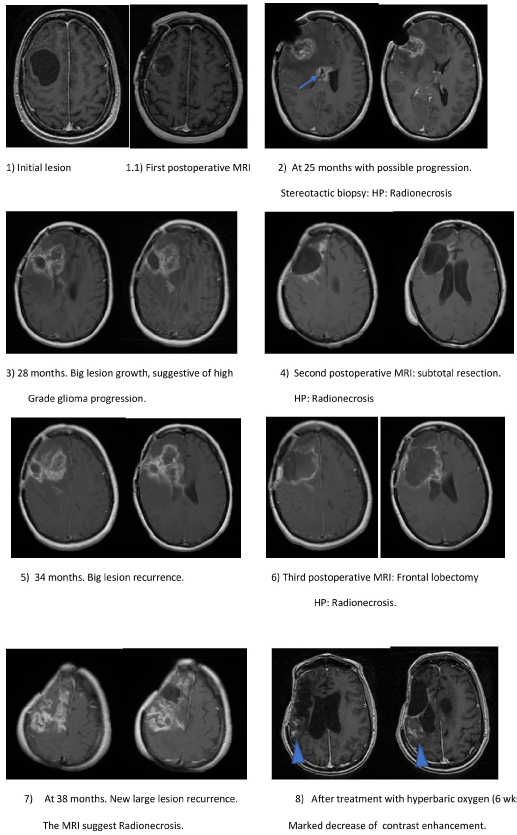 Figure 1: Primary lesion and radionecrosis recurrences in MRI, treated with surgeries.
Figure 1: Primary lesion and radionecrosis recurrences in MRI, treated with surgeries.
Initial nodule-cystic lesion with small nodular enhancement. 1.1) subtotal resection, HP: anaplastic gemistocytic astrocytoma, patient receives RT and CTx; 2) New large lesion at 25 months suggesting progression, HP: RN. A new second lesion in corpus callosum (arrow); 3) Growth of previous injuries, 28 months; 4) Subtotal resection, HP: RN; 5) A new giant recurrence at 34 months suggesting progression; 6) Subtotal right frontal lobectomy, HP: RN; 7) New giant recurrence at 38 months. Patient refuse surgery. MRI supports BRN, and all previous histopathology were BRN. Hyperbaric oxygen treatment is decided; 8) Very marked reduction of contrast enhancement at the end of treatment with hyperbaric oxygen chamber with minimum residual lesion.
View Figure 1
HP: Gemistocytic anaplastic astrocytoma, Ki-67 (25%). STUPP protocol is completed and received total dose: 60Gy; D.max: 65.91 Gy; D.min: 51.68 Gy; D average: 63.22 Gy. MRI follow-up every 3 months for 2 years were uneventful.
At 25 months, she presented headache and bradipsiquia, MRI demonstrates two new lesions in: corpus callosum and right frontal lobe, with contrast enhancement, without increased cerebral blood volume (rCBV), normal perfusion, highly suggestive of BRN (Figure 1.2). A stereotactic biopsy result is BRN (reactive gliosis and Ki67 < 3%) (Figure 2). The patient is treated with corticosteroids, improving symptoms.
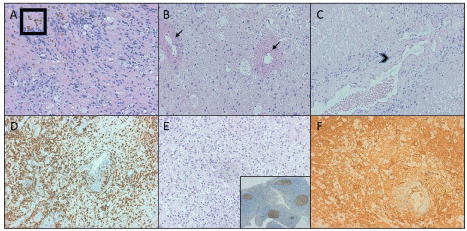 Figure 2: Histopathological findings in different surgeries.
Figure 2: Histopathological findings in different surgeries.
A) Marked histiocytic and lymphocytic infiltration at the periphery of the lesion accompanied of hemosiderin deposits (Box); B) Noninflammatory fibrinoid vascular necrosis (arrows) with cystic change and histiocytic infiltration; C) Coagulative necrosis and vascular hyalinization with luminal stenosis (arrow head); D) Marked histiocytic infiltration highlighted with CD68; E) Low proliferation index (Ki67). Microvascular proliferation, glomeruloide type. Control at the bottom inset; F) Reactive gliosis (highlighted with GFAP), histiocytic infiltrate and vascular hyalinization with luminal stenosis.
View Figure 2
At 28 months, presented severe left hemiparesis 2/5 (*). The MRI showed growth of lesions and increased cerebral edema, restricted diffusion and slight perfusion increase (2×) (Figure 1.3). The positron emission tomography (PET-CT) revealed a slight increase in FDG uptake suggesting tumor progression. Subtotal removal (Figure 1.4) is performed under 5-Aminolevulinic acid (5-ALA) fluoroscopy, without showing any fluorescence in the surgical field HP diagnosis was BRN (Figure 2). The patient has a marked clinical improvement.
At 34 months, she had progressive left hemiparesis 4/5. The MRI showed recurrence of the right frontal lesion, with significant associated edema, restricted diffusion, increased perfusion, elevated ratios of choline/creatine and choline/NAA; also having a prominent peak lipid and lactate, suggesting necrosis (Figure 1.5). The patient developed hemiplegia refractory to corticosteroids. A right frontal lobectomy (Figure 1.6) under 5-ALA was performed, showing absence of intraoperative fluorescence HP: Radionecrosis (Figure 2). The patient improved clinically.
At 38 months, the patient had once again left hemiparesis 3/5a. MRI showed edema and a new contrast enhancement in the surgical cavity. This time, the patient refused further surgery, and treatment was started with hyperbaric oxygen for 6 weeks, (Figure 1.7 and Fugure1.8). At February 2019 the patient is still alive with no recurrences.
aModified motor scale, the Great Britain Royal Medical Research Council.Differentiating between brain radiation necrosis and disease progression (high grade glioma) remains a challenge [1,4,7]. Severe BRN is a well-known and documented phenomenon [2,3,6,7]. However, recurrent radionecrosis in such a short period of time (a few months) and with such aggressiveness is extremely rare. The BRN can have a very aggressive behavior and even greater than a high-grade glioma (as in this case), with giant recurrences in a few months and being necessary to perform several surgeries and therefore increasing morbidity and mortality in these patients as in this case. The best way to treat BRN with a significant associated mass effect is surgical resection. The extent of resection is important in BRN and even more so in the setting of tumor progression. The use of 5-ALA is an easy way that could help differentiate BRN from a high-grade glioma during surgery, although it is not completely reliable. Hyperbaric oxygen therapy could be a valid option for the symptomatic and radiological control of BRN in the absence of a severe deficit, small recurrences and when the patient rejects further surgeries. We have seen a very good control on necrosis in this case up to 16 months and still no recurrences. Hyperbaric oxygen therapy has shown efficacy in treating radionecrosis in different organs and may also be useful in the brain, [8]. Antiangiogenic therapy such as Bevacizumab (vascular endothelial growth factor blocker [VEGF]) has also been used in treatment with BRN with good results [9].
Giant and recurrent cerebral radionecrosis in few months is infrequent and can have an aggressive behavior presenting important neurological deficits, large masses and severe edema that may require multiple surgeries to control their growth and improve clinical status. It can be confused with a high-grade brain tumor and behave as such. Proper control can allow a long-life expectancy. The treatment with hyperbaric chamber in this case has allowed good long-term control without side effects.