Obstetrics and Gynaecology Cases - Reviews
Bilateral Uterine Artery Chemoembolization with Methotrexate and Gelatine Foam for Caesarean Scar Pregnancy - Case Report
Krzysztof Pyra1*, Slawomir Wozniak2, Piotr Czuczwar2, Piotr Szkodziak2 and Michal Sojka1
1Interventional Radiology Department, University Hospital of Lublin, Poland
2Gynecology Department, University Hospital of Lublin, Poland
*Corresponding author: Krzysztof Pyra, Interventional Radiology Department and Neuroradiology, Jaczewskiego 8 Street, 20-954 Lublin, Poland, Fax: 0048 81 72 44 800, E-mail: k.pyra@poczta.fm
Obstet Gynecol Cases Rev, OGCR-1-003, (Volume 1, Issue 1), Case Report; ISSN: 2377-9004
Received: September 10, 2014 | Accepted: October 27, 2014 | Published: October 29, 2014
Citation: Pyra K, Wozniak S, Czuczwar P, Szkodziak P, Sojka M (2014) Bilateral Uterine Artery Chemoembolization with Methotrexate and Gelatine Foam for Caesarean Scar Pregnancy - Case Report. A Case Report. Obstet Gynecol Cases Rev 1:003. 10.23937/2377-9004/1410003
Copyright: © 2014 Pyra K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords
Caesarean scar pregnancy, Embolization, Methotrexate
Abbreviations
CSP: Cesarean Scar Pregnancy; UTACE: Uterine Artery Chemoembolization; MTX: Methotrexate; D&C: Dilatation and Curettage
Introduction
Caesarean scar pregnancy (CSP) is the rarest type of ectopic pregnancy. It is a life-threatening abnormal form of implantation of a gestational sac in the myometrium at the site of a previous Caesarean scar. CSP was first described by Larsen and Salomon in 1978. It is estimated that CSP constitutes about 6% of all ectopic pregnancies in patients with a history of at least one Caesarean section. The incidence of this pathology ranges from 1/1800 to 1/2200 pregnancies and its rate is 0.15% in women with previous Caesarean sections. CSP can lead to massive haemorrhages, uterine rupture, and disseminated intravascular coagulation (DIC) or death. Early diagnosis is essential for effective treatment to avoid life-threatening complications. Available data suggest that termination of pregnancy shortly after the diagnosis of CSP is the treatment of choice in the first trimester. Postponed treatment is usually associated with poor prognosis. [1-7].
Treatment options of CSP include dilatation and curettage (D&C) as well as excision of trophoblastic tissues using laparotomy or laparoscopy. There are literature case reports describing successful treatment of CSP by systemic and/or local administration of methotrexate (MTX) with subsequent D&C [8,9]. Classic surgery and conservative treatment can be considered the first-line treatment in many early, unruptured ectopic pregnancies. In cases of active bleeding, uterine artery chemoembolization (UTACE) with MTX can become the treatment of choice, particularly in young women who want to preserve fertility [1-5]. We present a case of haemorrhage due to Caesarean scar pregnancy successfully treated with UTACE.
Case Report
A 33-year-old woman at 8 weeks of gestation (7w 5d), gravida 2 (1 previous cesarean section), was referred to the hospital due to massive bleeding from the genital tract. On admission, the physical examination demonstrated the patient in good general health, blood pressure values - 112/77mm Hg, pulse - 72/min, respiratory rate - 17 breaths per minute. Bimanual examination revealed a softened and enlarged uterus of a size of about 7 weeks` gestation, the cervix was closed. Bleeding from the external os of the cervical canal was visible in the speculum examination. The laboratory results showed Hb -13.4 mg%, HCT – 40.6%, RBC- 4.51 ml, L – 7.5k/µl, total hCG– 78727mIU/ml, blood group O, Rh D - negative. Transvaginal ultrasound confirmed Caesarean scar pregnancy. Anteflexion of the uterine body was observed; foetal ovum structures were not visualized in the uterine cavity. A single gestational sac – GS-27mm (8w1d) containing one viable embryo (CRL – 12mm; 7w3d) and a yolk sac (YS – 5mm) were visible in the region of the uterine isthmus at the site of the Caesarean scar. Foetal heart rate (FHR) was 142/min. The trophoblast was located at the site of the previous Caesarean scar. The myometrium thickness at the site of ovum implantation was 1.5 mm. The Doppler scan revealed extremely rich vascularisation at the site of implantation. The adnexa appeared normal. No free fluid in the pouch of Douglas was visualised. The 3D virtual organ computer-aided analysis (VOCAL) disclosed the foetal ovum volume of 12.1ml, 3D vascularisation - VI (vascularisation index) -36.5; FI (flow index) - 43.6 VFI (vascularisation flow index) -24.1. A prophylactic dose of anti-D immunoglobulin was administered. The patient was qualified for chemoembolization.
The examination and the procedure were performed under local anaesthesia by puncturing the right femoral artery using the Seldinger technique. The 5F vascular sheath was inserted; the Pigtail catheter was placed in the abdominal aorta. Digital subtraction angiography of both internal iliac arteries was performed by an experienced interventional radiologist. Using the cross-over method, the 5Fr Robert catheter was moved to the opposite side. The uterine artery was selectively cannulated using the Progreate microcatheter; methotrexate 25mgmethotrexate was infused bilaterally(total 50 mg)administered in a slow bolus and the vessel was embolized using the gelatine foam (technical pearls: two screw syringes, three-way stopcock, gelatin sponge cut into small pieces, add contrast, mix it between the syringes through a stopcock) until stasis was achieved. The same procedure was carried out on the opposite side. The retention of contrast medium was visible within the uterus. Angiography repeated5 minutes after embolisation demonstrated lack of fresh contrasting blood inflow to both uterine arteries. The balloting contrasting medium confirmed the proper course of the procedure (Figure 1). Since D&C was planned, the vascular access was closed with the AngioSealoccluder. After treatment, the post-embolization syndrome occurred, consisting of fever, moderate lower abdominal pain and nausea, and lasted 1 day; pain was successfully controlled using a morphine pump. No severe complications were observed.
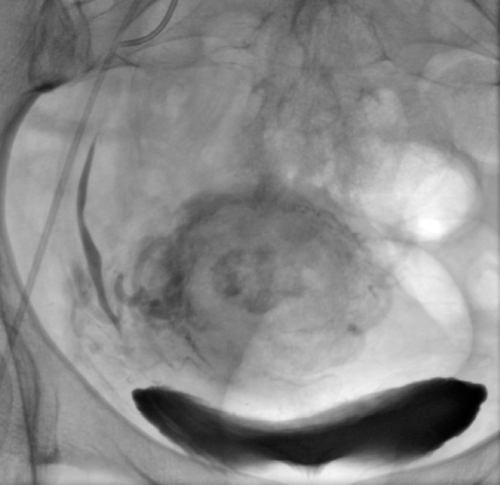 Figure 1: Stagnation of contrast medium in the uterus after chemoembolization
confirms the effectiveness of the procedure.
View Figure1
Figure 1: Stagnation of contrast medium in the uterus after chemoembolization
confirms the effectiveness of the procedure.
View Figure1
Total hCG determined after 24 h was 28105 mIU/ml. Blood test results were – Hb – 12.5mg%, HCT- 34.5%, RBC- 4.03ml, L – 9.04 k/µl. Ultrasonographic examination was repeated. The FHR was not visualized. Power Doppler examination and angio3D did not visualize vascularisation within the foetal ovum. The volume of the foetal ovum measured with VOCAL was 9 ml. The physical examination revealed minor bleeding from the cervical canal. The patient was qualified for suction curettage. The procedure was performed under general anaesthesia and ultrasound-guided (the transabdominal probe 3.5MHz). The 12mm Hegar dilator was easily inserted into the cervical canal. The products of conception (necrotic tissues) were removed. Intra- and post-procedure bleeding was minimal. After curettage 50µg anti-D immunoglobulin was administrated. After 12 hours, blood tests were repeated showing HB -11.3 mg%, HCT -34.5%, RBC -3.57ml, L-8.87 k/µl; hCG re-determined on the following day was 9718mIU/ml and dropped to 4606mIU/ml after the next 24h. Forty-eight hours after the procedure another transvaginal ultrasound examination showed an empty uterine cavity and no pathological echoes at the site of post-cesarean scar. The physical examination disclosed a uterus of normal size and trace spotting from the cervical canal. The patient was discharged from hospital in a good general condition. Total hCG re-determined two weeks later was 36IUm/ml; its level decreased to 3IUm/ml after the next 7 days. On post-procedure day 31, normal menstruation occurred.
Discussion
Caesarean section is a risk factor of ectopic pregnancy, placental pathology and massive bleeding in future pregnancies [6]. Since the first case report of Caesarean scar pregnancy published by Larsen and Salomon in 1978, its incidence has been increasing. The pathogenesis of CSP has not been fully elucidated.
To date, Caesarean scar pregnancy and placenta percreta have been regarded as distinct entities. The most recent data indicate that they are the continuum of the same pathology rather than separate diseases. In CSP, the conception product can penetrate the endometrium through the microscopic dehiscences in the Caesarean scar [7]. If the wait-and-see attitude is accepted in the first trimester of CSP, it is most likely to evolve into placenta percreta. In almost each case, this leads to postpartum haemorrhage and requires a hysterectomy. Early diagnosis and appropriate interventions are likely to substantially improve the prognosis [10-12].
The suggested sonographic diagnostic criteria of CSP include an empty uterine cavity, empty cervical canal, development of the gestational sac in the anterior part of the uterine isthmus, and blood flow around the gestational sac. The differential diagnosis of CSP and cervico-isthmic pregnancy is based on the absence of physiological myometrium between the bladder and the gestational sac in scar pregnancies [2-4].
The diagnosis of CSP is easier in the first trimester of pregnancy. In the second and third trimester, CSP can be misdiagnosed as low implantation of the intrauterine pregnancy, cervical pregnancy or inevitable abortion [13,14]. MRI can be useful in cases of ultrasound- based doubts [13,15,16]. The time interval from the last Caesarean section is not defined as a risk factor of CSP. According to literature data, the time in question ranges from 6 months to 12 years. No significant correlations between the number of Caesarean sections and the risk of CSP were observed [15,17,18]. To date, numerous invasive and conservative methods of CSP treatment have been described. Some authors suggest minimally invasive methods, such as hysteroscopy, laparoscopy or D&C as the treatment options preserving fertility [17,19-22]. Theclassic method of treatment widely described in literature is systemic administration of MTX followed by D&C. The drawbacks of this method include the exposure to high doses of a chemotherapeutic as well as possible significant peri-procedural bleeding. In many cases, this procedure has to be converted to laparotomy, often leading to hysterectomy. The watch and wait attitude is not recommended due to a high risk of uterine rupture. Despite increasing incidence rates of Caesarean scar pregnancy, no universal guidelines for its management and treatment have been suggested. It is recommended to terminate the pregnancy in the first trimester to prevent life-threatening complications [7,18-27].
Lanand colleagues presented a series of 79 CSP cases effectively treated with the method described above. They suggested suction curettage to be performed 24 -48 hours after bilateral uterine artery chemoembolization. No bleeding or other life-threatening complications were observed. The mean blood loss during suction curettage after embolization of uterine arteries was small, about 30 ml [8].
In Bo Zhang et al. follow-up, uterus was preserved in 14 of the 15 patients. No serious adverse effects were observed in these patients. Their menstruation resumed an average of 2 months later [28].
Also Yang XY et al. in retrospective cohort study, where total of 66 women diagnosed with caesarean scar, were treated with dilation and curettage (D&C) in 11 patients, systemic MTX in 17 patients, and UAE and local MTX in 38 patients, suggest that UAE combined with local MTX is of benefit to women wishing to preserve fertility, and is suitable for use as the primary treatment for caesarean scar pregnancy. Moreover significantly shorter duration of hospital stay was observed in group with UTACE compared with systemic MTX [29].
Licong Shen et al. described a group of 46 patients treated with chemoembolization and local administration of methotrexate. According to their findings, bilateral uterine artery chemoembolization and MTX administration seemed to be a safe and effective CSP treatment method, with fewer complications than other methods [30].
Pisco et al. present a series of 74 women who wanted to become pregnant, 44 of them became pregnant after UFE. Study is significant because it shows comparable fertility rates 59.5% % vs 58,2 % fertility rate after myomectomy from Sudik R et al. study. Thus, embolisation allows having normal pregnancies with similar complication rates as the general population. In Pisco study UAE was made with non-resorbable particles, our case was done with resorbable gelatin foam, which gives us even greater opportunities to preserve fertility [31,32].
During bilateral uterine artery chemoembolization, methotrexate is administered to the vessel directly supplying the gestational foci subsequently embolized. The resultant artery closure hinders the blood flow at the site of CSP so that even a low dose of MTX results in a high local drug concentration, which together with local ischaemia induces effective therapeutic effects. While the total dose of MTX is decreased, its local concentration increases and its action within the gestational foci are prolonged, leading to more effective embryocide. The additional modification, i.e. the use of the absorbable embolizing material, increases the chances for fertility preservation.
Our case demonstrates the successful use of local MTX application with subsequent closure of uterine arteries with absorbable gelatin sponge for Caesarean scar pregnancy treatment.
In conclusion, UTACE with the use of absorbable embolizing material seems to be an effective and safe method.It is characterized by low invasiveness that enables preservation of fertility and additionally reduces the risk of bleeding during D&C. It might be a life-saving procedure in cesarean scar pregnancy hemorrhages.
Contribution to Authorship
Krzysztof Pyra; MichalSojka - Carry out the embolization procedure and wrote radiological part of report
SlawomirWozniak - Recognized CSP, ultrasound diagnostics, qualified for embolization
Piotr Czuczwar - Wrote gynecological part of report
Piotr Szkodziak - Post-operative care
The patient has consented to the publication of the description of her case Treatment, care and embolization funded by the national health fund.
References
-
Maymon R, Halperin R, Mendlovic S, Schneider D, Vaknin Z, et al. (2004) Ectopic pregnancies in Caesarean section scars: the 8 year experience of one medical centre. Hum Reprod 19: 278-284.
-
Jurkovic D, Hillaby K, Woelfer B, Lawrence A, Salim R, et al. (2003) First-trimester diagnosis and management of pregnancies implanted into the lower uterine segment Cesarean section scar. Ultrasound Obstet Gynecol 21: 220-227.
-
Fylstra DL (2002) Ectopic pregnancy within a cesarean scar: a review. Obstet Gynecol Surv 57: 537-543.
-
Rotas MA, Haberman S, Levgur M (2006) Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol 107: 1373-1381.
-
Yan CM (2007) A report of four cases of caesarean scar pregnancy in a period of 12 months. Hong Kong Med J 13: 141-143.
-
Herman A, Weinraub Z, Avrech O, Maymon R, Ron-El R, et al. (1995) Follow up and outcome of isthmic pregnancy located in a previous caesarean section scar. Br J Obstet Gynaecol 102: 839-841.
-
Godin P, Bassil S, Donnez J (1997) An ectopic pregnancy developing in previous caesarean section scar. Fertil Steril 67: 398-440.
-
Fadhlaoui A, Khrouf M, Khemiri K, Nouira K, Chaker A, et al. (2012) Successful conservative treatment of a cesarean scar pregnancy with systemically administered methotrexate and subsequent dilation and curettage: a case report. Case Rep Obstet Gynecol 248564.
-
Lan W, Hu D, Li Z, Wang L, Yang W, et al. (2013) Bilateral uterine artery chemoembolization combined with dilation and curettage for treatment of cesarean scar pregnancy: A method for preserving the uterus. J Obstet Gynaecol Res 39: 1153-1158.
-
Rosen T (2008) Placenta accreta and cesarean scar pregnancy: overlooked costs of the rising cesarean section rate. Clin Perinatol 35: 519-529, x.
-
Sinha P, Mishra M (2012) Caesarean scar pregnancy: a precursor of placenta percreta/accreta. J Obstet Gynaecol 32: 621-623.
-
Timor-Tritsch IE, Monteagudo A (2012) Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. Am J Obstet Gynecol 207: 14-29.
-
Sadeghi H, Rutherford T, Rackow BW, Campbell KH, Duzyj CM, et al. (2010) Cesarean scar ectopic pregnancy: case series and review of the literature. Am JPerinatol 27: 111-120.
-
McKenna DA, Poder L, Goldman M, Goldstein RB (2008) Role of sonography in the recognition, assessment, and treatment of cesarean scar ectopic pregnancies. J Ultrasound Med 27: 779-783.
-
Ahmadi F, Zafarani F, Haghighi HB, Niknejadi (2010) Ectopic Pregnancy in Cesarean Section Scar: A Case Report. Int J Fertil Steril 4: 140-142
-
Ash A, Smith A, Maxwell D (2007) Caesarean scar pregnancy. BJOG 114: 253-263.
-
Seow KM, Huang LW, Lin YH, Tsai YL, Hwang JL (2004) Cesarean scar pregnancy: issues in management. Ultrasound Obstet Gynecol 23: 247-253.
-
Seow KM, Cheng WC, Chuang J, Lee C, Tsai YL, et al. (2000) Methotrexate for cesarean scar pregnancy after in vitro fertilization and embryo transfer. A case report. J Reprod Med 45: 754-757.
-
Hwu YM, Hsu CY, Yang HY (2005) Conservative treatment of caesarean scar pregnancy with transvaginal needle aspiration of the embryo. BJOG 112: 841-842.
-
Weimin W, Wenqing L (2002) Effect of early pregnancy on a previous lower segment cesarean section scar. Int J Gynaecol Obstet 77: 201-207.
-
Deb S, Clewes J, Hewer C, Raine-Fenning N (2007) The management of Cesarean scar ectopic pregnancy following treatment with methotrexate--a clinical challenge. Ultrasound Obstet Gynecol 30: 889-892.
-
Condous G (2009) Ectopic pregnancy: challenging accepted management strategies. Aust N Z J Obstet Gynaecol 49: 346-351.
-
Lai YM, Lee JD, Lee CL, Chen TC, Soong YK (1995) An ectopic pregnancy embedded in the myometrium of a previous cesarean section scar. Acta Obstet Gynecol Scand 74: 573-576.
-
Lam PM, Lo KW, Lau TK (2004) Unsuccessful medical treatment of cesarean scar ectopic pregnancy with systemic methotrexate: a report of two cases. Acta Obstet Gynecol Scand 83: 108-111.
-
Kang SY, Park BJ, Kim YW, Ro DY (2011) Surgical management of cesarean scar ectopic pregnancy: hysterotomy by transvaginal approach. Fertil Steril 96: e25-28.
-
Litwicka K, Greco E (2011) Caesarean scar pregnancy: a review of management options. Curr Opin Obstet Gynecol 23: 415-421.
-
Tulpin L, Morel O, Malartic C, Barranger E (2009) Conservative management of a Cesarean scar ectopic pregnancy: a case report. Cases J 2: 7794.
-
Zhang B, Jiang ZB, Huang MS, Guan SH, Zhu KS, et al. (2012) Uterine artery embolization combined with methotrexate in the treatment of cesarean scar pregnancy: results of a case series and review of the literature. J Vasc Interv Radiol 23: 1582-1588.
-
Yang XY, Yu H, Li KM, Chu YX, Zheng A (2010) Uterine artery embolisation combined with local methotrexate for treatment of caesarean scar pregnancy. BJOG 117: 990-996.
-
Shen L, Tan A, Zhu H, Guo C, Liu D, et al. (2012) Bilateral uterine artery chemoembolization with methotrexate for cesarean scar pregnancy. Am J Obstet Gynecol 207: 386.
-
Pisco JM, Duarte M, Bilhim T, Cirurgião F, Oliveira AG (2011) Pregnancy after uterine fibroid embolization. Fertil Steril 95: 1121.
-
Sudik R, Hüsch K, Steller J, Daume E (1996) Fertility and pregnancy outcome after myomectomy in sterility patients. Eur J Obstet Gynecol Reprod Biol 65: 209-214.





