Endometrial cancer (EC) is the most common gynecological malignancy in developed countries with a lifetime risk of 3% for women living in the United States [1]. Women younger than 45 years of age represent 7% of endometrial cancer cases [2]. Type 1 endometrioid adenocarcinoma is the most common type. Endometrial intraepithelial neoplasia (EIN) is a precursor lesion that carries a risk of concomitant invasive disease in up to 40% of patients [3,4].
Risk factors for type 1 endometrial cancer and EIN include unopposed estrogen exposure such as in polycystic ovary syndrome, menstrual irregularities, nulliparity and obesity [5]. These risk factors can be encountered in younger women seeking infertility treatment. While surgical staging that includes hysterectomy and bilateral salpingo-oophorectomy remains to be the definitive management, conservative management could be an acceptable option for patients who desire fertility sparing treatment [6-8]. Current conservative management is limited to progestogen therapy. Levonorgestrel IUD and megestrol acetate are the two most used progestogens in the clinical setting. Regression of EIN and EC ranges between 70-90% [9-11]. The choice of which progestogen therapy to recommend is currently not clear, but they all seem to have positive outcomes [12].
Our patients in this case series presented to a private reproductive endocrinology office for infertility (2008-2018). During infertility work up, the diagnosis of endometrial cancer or precursor lesions was established after a diagnostic hysteroscopy dilatation and curettage (D&C) (Figure 1). The patients had variable characteristics contributing to their infertility. The aim of this case series is to discuss the reproductive outcomes of six patients who underwent in vitro fertilization (IVF) following conservative management.
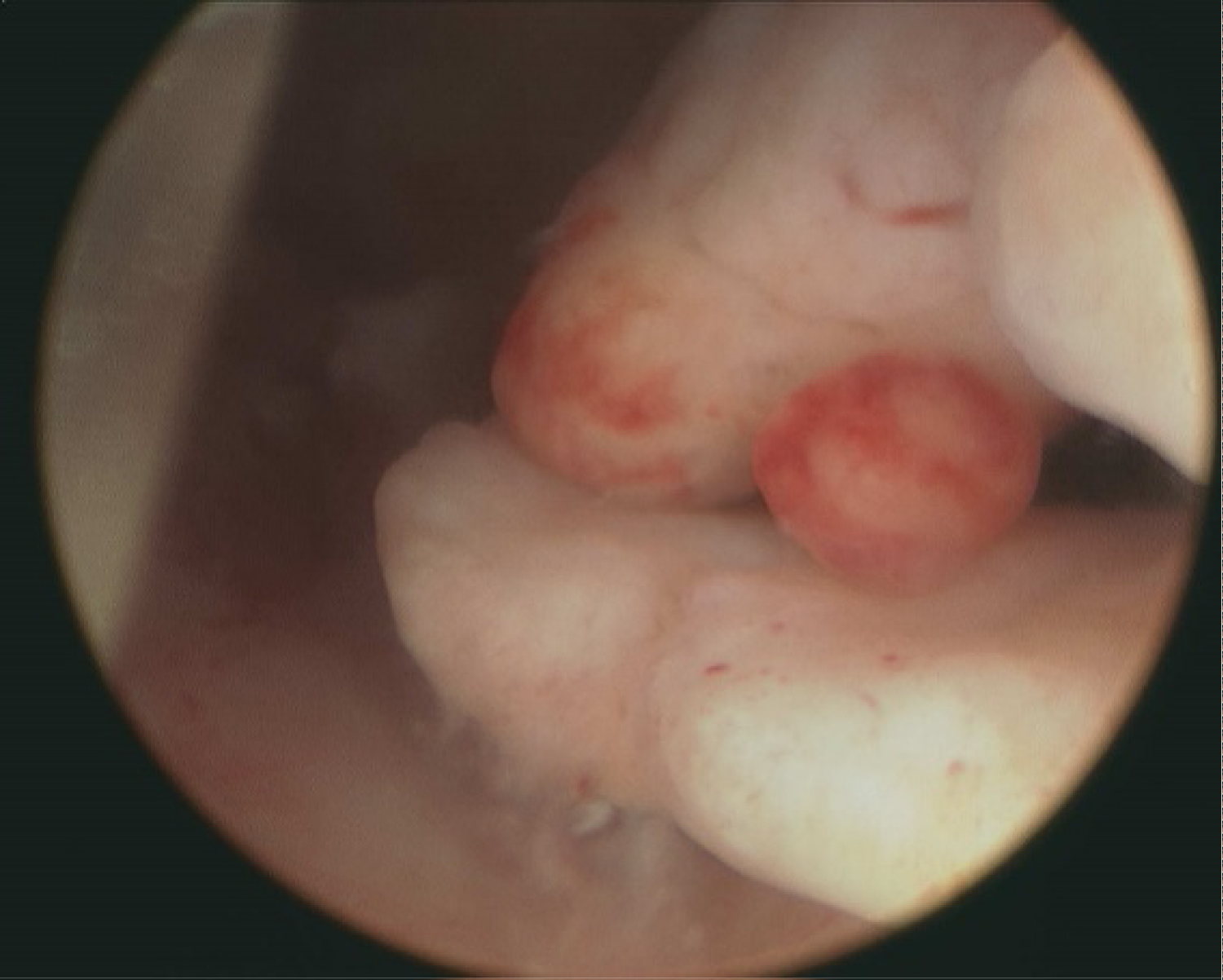 Figure 1: Multiple endometrial polyps on hysteroscopy in patient # 6 with pathology report showing endometrial cancer.
View Figure 1
Figure 1: Multiple endometrial polyps on hysteroscopy in patient # 6 with pathology report showing endometrial cancer.
View Figure 1
This is a 32-year-old nulligravid with a 10-year history of primary infertility secondary to polycystic ovary syndrome. During her initial fertility workup, filling defects were noted during saline hysterography suspicious for endometrial polyps. She then had a hysteroscopic polypectomy and uterine septum revision. The histopathology report displayed the diagnosis of focally atypical, endometrial hyperplasia with extensive squamous morular metaplasia. The patient was referred to Gynecologic Oncologist as she still desired to retain her uterus for future fertility. She was started on Megace 80 mg b.i.d for 6 months. She had a D&C every 3-6 months to assess for treatment response vs any sign of progression. She had an appropriate response and thirteen months later began treatment with assisted-reproductive technology. She had a total of two IVF cycles. The first was a “minimal stimulation IVF” protocol which was converted to natural intercourse due to poor ovarian response and a thin endometrial lining of 6 mm. She did not conceive. Second cycle was a traditional antagonist protocol with 2 blastocysts transferred. Unfortunately, this cycle was unsuccessful in achieving a pregnancy. She plans to attempt IVF treatment for fertility in the future.
A 33-year-old nulligravid with a 6-year history of primary infertility presented with irregular cycles along with a history of endometriosis. She underwent laparoscopic lysis of adhesions, hysteroscopic polypectomy and septum revision. The pathology results showed complex atypical hyperplasia for which she was prescribed Megace 80 mg b.i.d. Eighteen months after her diagnosis she had a fresh IVF cycle resulting in 11 frozen embryos and 2 embryos which were transferred. Sadly, her pregnancy resulted in a heterotopic pregnancy with an intrauterine blighted ovum and right-sided tubal pregnancy treated via salpingectomy. Upon repeat D&C, her endometrial evaluation showed benign hyperplasia without atypia or malignancy. She underwent a frozen cycle, but unfortunately no embryos survived the thawing process. She ultimately chose to proceed with hysterectomy. Her time from diagnosis to hysterectomy was 30 months.
A 33-year-old Gravida 1 Para 0 with history of spontaneous miscarriage. She presented with a 3.5 year-history of secondary infertility secondary to uterine and male factor. She had a previous unsuccessful fresh IVF-ET cycle in another facility and had frozen embryos remaining. She had a hysteroscopic division of an incomplete uterine septum and D&C. Her pathology report returned showing grade 1 (FIGO staging) endometrial adenocarcinoma. A Mirena IUD was placed for 3 months and was removed after confirming absence of malignancy via endometrial sampling. 11 months following her initial diagnosis, she underwent a frozen IVF cycle that resulted in a Dichorionic-Diamniotic twin pregnancy. She was induced at 33-week gestation for preeclampsia and delivered vaginally. The newborns required NICU admission for 2 weeks, but did well afterwards.
A 38-year-old nulligravid with a 1-year history of primary infertility secondary to polycystic ovary syndrome, endometriosis as well as her age and ovarian reserve. She had a laparoscopic left ovarian cystectomy, lysis of adhesions and hysteroscopic polypectomy. The pathology results showed grade 1 (FIGO staging) endometrial adenocarcinoma and was treated with Megace 40 mg q.id. Repeat D&C 6 months later revealed simple hyperplasia. She then transferred her care to another infertility facility. She underwent a total of 3 cycles of clomid and another 3 cycles of clomid and Intrauterine insemination without success. Thirty-one months after her initial cancer diagnosis, she was diagnosed with recurrence of endometrial adenocarcinoma and she was given a repeat course of Megace 40 mg qid. One year after her recurrent diagnosis, she had hysteroscopic polypectomy and the pathology report showed no evidence of malignancy. Thirteen months after her recurrence, she had two unsuccessful IVF cycles, followed by a 3rd successful IVF cycle that resulted in live birth, at age 42. At age 45 years and after endometrial sampling ruled out malignancy, she was advised to have IVF treatment with donor egg program in view advanced maternal age but she declined. She had 4 more IVF trials, two were cancelled due poor ovarian response and two cycles ending in single embryo transfer without success. She was advised to have a hysterectomy to avoid recurrence of endometrial cancer.
This is a 38-year-old Gravida 4 para 0 with 8-year history of secondary infertility with previous intrauterine insemination resulting in four spontaneous miscarriages. She had a possible male factor component in her infertility history as well. On initial uterine evaluation, endometrial polyps were diagnosed and removed via hysteroscopy. She was also found to have an incomplete uterine septum, which was not diagnosed in the past. The septum was divided hysteroscopically at the same setting. Her pathology report revealed well-differentiated grade 1 (FIGO staging) endometrial adenocarcinoma. She was started on Megace 40 mg qid. She had repeated endometrial sampling every 3 months with D&C to rule out malignancy recurrence and assess her treatment response. Six months after her diagnosis, she underwent a fresh IVF fresh cycle resulting in 7 embryos. Preimplantation genetic screening revealed that all embryos were chromosomally abnormal, and no embryos were transferred. Since her most recent endometrial tissue biopsy showed endometrial hyperplasia with atypia, she chose to proceed with hysterectomy rather than attempt conservative management again.
This is a 34-year-old female with a 7-year history of primary infertility secondary to polycystic ovary syndrome. She had multiple uterine polyps on imaging and hysteroscopy. She had hysteroscopic removal of her polyps and subsequently was diagnosed with grade 1 (FIGO staging) endometrial adenocarcinoma on pathology. She was treated with Megace 40 mg qid for 3 months total before having a repeat hysteroscopy with negative pathology results. She then conceived via IVF and had a twin pregnancy that resulted in a preterm birth around 30 weeks gestation. Both newborns required NICU admission but eventually did well.She was on combined oral contraceptives following her pregnancy. She returned to our facility for a second infertility evaluation, but ultimately decided to proceed with hysterectomy two years following her delivery.
Table 1 illustrates the characteristic summary of the six cases. The age range at diagnosis was 32-38 years (mean 34.6). Average BMI was 37.3 Kg/m2. Four out of six patients had primary infertility. The remaining 2 patients had secondary infertility, but no history of successful live births. Shared characteristics included polycystic ovary syndrome (PCOS) in 50% of patients. Pathology results of endometrial sampling showed EIN in two patients and grade 1 endometrioid adenocarcinoma in the remaining four patients (Table 1). Following high-dose progestogen treatment with megestrol acetate (5/6) or Mirena (1/6), patients were recommended to have IVF-ET treatment. Pregnancy and delivery rates per patient were 66.7% and 50% respectively (Figure 2). Pregnancy and delivery rates per ET were 80% and 60% respectively (Figure 2). One patient (Patient # 2) had a heterotopic pregnancy and no live birth (Table 2). Two of the live births were twin gestation (50%) (Figure 2).
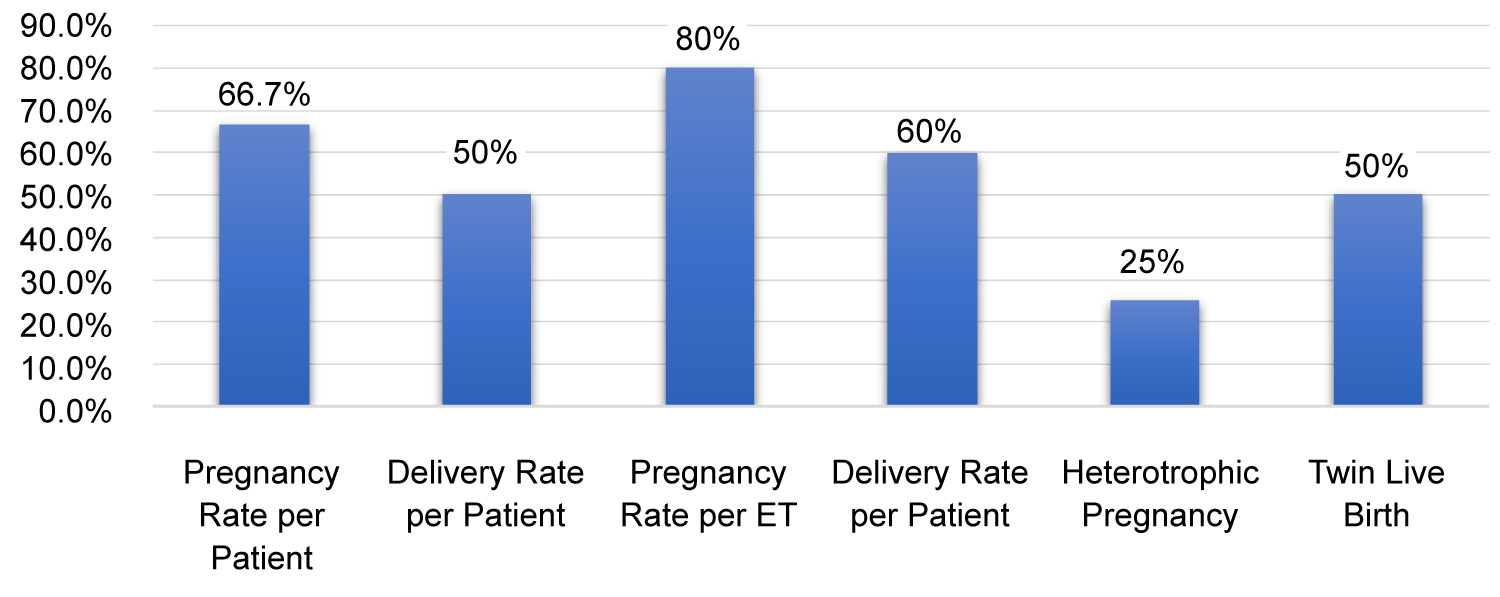 Figure 2: Reproductive outcomes of IVF-ET after Conservative treatment for endometrial cancer and endometrial intraepithelial neoplasia.
View Figure 2
Figure 2: Reproductive outcomes of IVF-ET after Conservative treatment for endometrial cancer and endometrial intraepithelial neoplasia.
View Figure 2
Table 1: Case summaries. View Table 1
Table 2: Summary of IVF-ET results. View Table 2
There is continued interest in conservative management of endometrial carcinoma, particularly in the population of patients wishing to maintain fertility. However, this clinical scenario is still a rare occurrence as premenopausal patients are less impacted by endometrial carcinoma when compared to their postmenopausal counterparts. The current literature is insufficient to provide specific recommendations guiding progestin therapy for the infertile patient. This can present unique challenges when counseling the patient regarding expectations and options following conservative therapy. There are few studies in the literature that discuss fertility outcomes following conservative management. At our institution, we present six patients who were diagnosed with endometrial carcinoma precursor lesions or grade 1 carcinoma who were treated with progestogen therapy and underwent assisted reproductive technology due to primary or secondary infertility.
The limitations of conservative therapy are widely expressed in the literature. We sought to focus on data that include patients with infertility as their primary rationale for seeking conservative therapy. Two large studies displayed promising reproductive outcomes following progestogen treatment. Gallos, et al. performed a systematic review and meta-analysis of 451 women that had fertility-sparing treatment for endometrial carcinoma or complex hyperplasia with atypia. Assisted fertility treatment was performed for 145 patients with a resulting live birth rate of 39.4% [9]. The remaining 309 cases were assumed to be seeking conception naturally and had a live birth rate of 14.9% [9]. This study encouraged both attempting conception with assisted reproductive technology or naturally due to low recurrence rates and promising live birth rates [9]. The authors emphasized immediate need for IVF-ET treatment in order to reduce time to pregnancy and reduce the risks of recurrence [9]. In addition, IVF-ET is associated with higher pregnancy and delivery outcomes much like our approach to the patients in our case series [9]. Wei, et al. conducted a review and meta-analysis looking into the live birth rates after different treatment modalities used after conservative management [13]. Live birth rates were 20%, 14%, 35% when oral progestin (medroxyprogesterone acetate and megestrol acetate), IUD and progestin plus IUD were used respectively [13].
In contrast, reproductive outcomes display more discouraging results in a recent large cohort study conducted in 2019 which included 421 patients diagnosed with endometrial cancer and complex endometrial hyperplasia with atypia who received fertility-sparing treatment [14]. The resulting live birth rate was only 11.6% [14]. Although only 15.5% of the cases were treated with assisted fertility services, 50% of the total live birth rate resulted from these cases [14]. They proposed that women affected by endometrial carcinoma and/or its precursors have inherent risks for lower reproductive potential as a result of their disease [14]. However, the purpose of these studies was not to specifically evaluate patient outcomes in the setting of infertility. In addition, the above studies cannot delineate between patients who chose conservative therapy for the sole indication of pregnancy. This alone can impact the reported live-birth rate and success in achieving pregnancy following treatment of endometrial carcinoma and complex hyperplasia with atypia.
On a smaller scale, there are reports of pregnancy outcomes concentrating on infertile patients which more strongly reflect the implications of our case series. Dursun, et al. investigated pregnancy outcomes in 41 patients with endometrial carcinoma. The pregnancy rate among the 31 patients who were actively seeking pregnancy was 41.9%. Conception occurred naturally in 2 (15.4%) patients and via assisted reproductive technologies in 11 (84.6%) patients [15]. These studies support the notion that infertility services maximize the chance of a successful pregnancy while shortening the interval from disease remission to conception. When considering IVF treatment along with the impact of endometrial carcinoma on fertility outcomes, Elizur, et al. evaluated eight infertile patients and the success of IVF-ET in the setting of endometrial adenocarcinoma [12]. A large proportion of their patients had anovulatory cycles, which is comparable to our patient population [12]. Out of 8 patients who underwent IVF-ET, six conceived. they noted a 28% pregnancy rate per cycle and 29% pregnancy rate per embryo transfer. 50% of patients (4/8) delivered healthy infants [12]. It is also worthwhile to note that the treatment effects may have an impact on reproductive potential during ART [12]. Some studies found that endometrial thickness itself can affect implantation rates [16,17]. Fujimoto, et al. found that transferring more embryos resulted in comparable live-birth outcomes to their controls [16]. To our knowledge, there are no specific guidelines in terms of the number of embryos to be transferred in such patients to improve pregnancy rates. One case series illustrated that patients required more IVF cycles when the endometrial lining measured less than 8 mm but with comparable live birth rates [12].
Our results are supported by the findings of similar infertility studies mentioned above [12,15]. Our study limitations include a small sample size, lack of long-term follow-up following infertility treatment and possible confounders. The strengths of our case series include our patient population whose characteristics may be generalizable to the premenopausal population who are affected by endometrial carcinoma but who wish to pursue pregnancy.
In conclusion, conservative management of endometrial carcinoma and its precursor lesions followed by IVF-ET have favorable outcomes in patients desiring fertility. Previous studies have reported that assisted reproductive technology was superior to achieving a pregnancy when compared to spontaneous conception [18]. We believe that careful selection of appropriate candidates for expectant management, along with thorough counseling and shared decision making is crucial to achieve the most optimal outcomes. We especially recommend the use of IVF, to shorten the duration between disease diagnosis and definitive management with hysterectomy in order to reduce risks of disease recurrence or progression. Further studies will expand the available literature and allow us to better predict the likelihood of positive reproductive outcomes. Further research is needed to provide established guidelines specific to IVF such as the optimal number of embryos transferred and risks of disease recurrence. In addition, specific research may be beneficial investigating the disease impact as well as treatment modalities upon endometrial thickness which can also impact pregnancy success rates.