Disorders of the vulva have characteristically received less attention than disorders of the cervix and other reproductive organs. There are a number of challenges in diagnosing vulvar neoplasia (pre-invasive and invasive vulvar disease) in Cameroon and other low- and middle-income countries (LMICs). This is primarily because the early signs and symptoms of vulvar neoplasia are non-specific and are predominantly characterized by pruritus vulvae, which can easily be mistaken for vulvar candidiasis. This leads to delay in getting appropriate treatment, with associated suffering, increased expenditure, progression of the disease, and poor prognosis.
This paper presents four case studies of women with vulvar neoplasia diagnosed by the Women's Health Program of the Cameroon Baptist Convention Health Services, a large faith-based health organization in Cameroon. These case reports demonstrate the challenges in diagnosing vulvar neoplasia in LMICs and highlight the importance of thoroughly evaluating vulvar symptoms and signs, such as pruritus, hypopigmentation, and leukoplakia with histopathology.
In Cameroon, West Africa, as in other low- and middle-income countries (LMICs), accurately diagnosing vulvar neoplasia is challenging, because it is relatively rare, with vulvar cancer accounting for only 3-5% of malignancies in women, affecting 1-2 women per 100,000 women yearly [1]. Human papilloma virus (HPV) causes 40-48%, and the rest are not associated with HPV [2]. Because vulvar neoplasia is less prevalent than other diseases associated with HPV such as cervical and oral cancers [3], there is minimal literature about its risk, diagnosis, and treatment.
Similarly, to cervical cancer, vulvar cancer begins with a premalignant or preinvasive phase called vulvar intraepithelial neoplasia (VIN) which later progresses into an invasive vulvar cancer (IVC) [2]. Unlike cervical cancer, for which there are various screening protocols, there is no specific screening test for vulvar cancer [4]. Cervical intraepithelial neoplasia (CIN) is the premalignant stage of cervical cancer and is completely asymptomatic [5]. However, VIN is usually symptomatic, with the primary and most predominant symptom being pruritus [2,4,6]. This symptom can easily be confused with vulvar candidiasis and, if pruritis is persistent, mislead clinicians to prolong treatment with antifungals without considering further diagnostic tests.
Unlike HPV associated vulvar neoplasia occurring in younger women, the non-HPV associated vulvar neoplasia generally occurs in the postmenopausal population [2,7]. One of the risk factors for non-HPV associated vulvar neoplasia is lichen sclerosus, an auto-immune disease affecting mostly postmenopausal women. It is characterized by vulvar discoloration and/or pruritus [7,8]. The symptoms of VIN and lichen sclerosus are very similar and provide a challenge in clinical diagnosis. The gold standard for diagnosing VIN, IVC, or lichen sclerosus is histopathology [6,9]. Therefore, it is paramount to perform biopsy on any vulvar lesions characterized by persistent pruritus or discoloration.
There have been some debates in the classification of VIN. Similar to CIN which is subcategorized into CIN1, 2, and 3 where severity increases from CIN1 to CIN3, VIN was also previously classified into three grades: VIN1, 2, and 3, with VIN1 being the mildest form, and VIN3 being the most severe. In 2004, the International Society for the Study of Vulvovaginal Disease (ISSVD) changed the classification to simply VIN representing VIN2/3, considering VIN1 as normal or of no clinical significance [4,10]. Later, in 2015, the ISSVD changed the classification to vulvar squamous intraepithelial lesion (SIL) and created two sub-categories; vulvar low-grade squamous intraepithelial lesion (vulvar LSIL) which represents HPV infection or flat condyloma and vulvar high-grade squamous intraepithelial lesion (vulvar HSIL) which represents the actual VIN [11]. However, the classifications are used indiscriminately in literature with many authors still using the VIN 1, 2, & 3 as classifications. In Cameroon, most pathologists report their vulvar histopathology findings using the VIN 1, 2, & 3 classification.
The Cameroon Baptist Convention Health Services (CBCHS) is a large faith-based organization in Cameroon, West Africa, that operates a system of 82 health facilities located in six of the 10 regions of Cameroon. The CBCHS runs a comprehensive nurse-led cervical cancer prevention program called the Women's Health Program (WHP) in 10 of its sites. WHP has screened over 80,000 women for cervical cancer in 10 years using a method called digital cervicography (DC) which serves as an added layer of screening to visual inspection with acetic acid (VIA) and visual inspection with Lugol's iodine (VILI) [12]. In DC, we use a digital camera with a macro-conversion lens or Samsung Galaxy cell phone to project magnified images of the cervix before and after VIA and VILI onto a monitor screen visible to both client and provider, take permanent photographs of the cervix, and, match them through a unique WHP identification number to the client's medical history and physical examination in the WHP database [12]. Before undergoing cervical cancer screening, the woman signs a consent permitting the provider to take and store photographs of her cervix for use in teaching and presentations. Because WHP is primarily concerned with cervical cancer prevention, every woman examined for a vulvar complaint is encouraged to also have cervical cancer screening, if she has not already done so.
Generally, WHP examines women for vulvar neoplasia using the following protocol. First, the vulva is inspected with the naked eye prior to application of any chemicals. Then a 5% acetic acid is applied for five minutes and inspected with the naked eye (VIA). If VIA is positive, we apply a 1% toluidine blue solution (toluidine blue test, or TBT) to further delineate abnormalities. Areas that stain white with acetic acid and/or blue with toluidine blue are considered positive for VIN [13]. We take photographs of the vulva before and after VIA and TBT and, as with cervical photographs, archive them in the WHP database in the same way we archive cervical photos.
We perform Keyes dermatological punch biopsies under local anesthesia on vulvar lesions that are suspicious for VIN or IVC. The specimen is placed in a 10% formalin solution and shipped for histopathology to a specialized facility (Yaoundé Gyneco-Obstetrics and Pediatric Hospital, Buea Regional Hospital Annex, or Mbingo Baptist Hospital). The WHP performed 60 vulvar biopsies between 2007 and 2016 (Table 1).
Table 1: Summary of vulva biopsies performed by the WHP. View Table 1
The following section of this paper presents four case studies illustrating the challenges in diagnosing vulvar neoplasia in Cameroon.
A 30-year-old G3P3 HIV negative woman was seen at the WHP clinic at Banso Baptist Hospital with a history of prolonged, intense pruritus vulvae of increasing severity. She had been treated for vulvar candidiasis several times to no avail and was then referred to WHP. At WHP, her cervical cancer screening was normal. The vulvar examination showed excoriation and acetowhite changes, especially on the labia majora (Figure 1). The histopathology showed VIN 3. WHP referred her to a surgeon, who successfully treated her with simple vulvectomy, which resolved the pruritus vulvae.
 Figure 1: VIN3; notice acetowhite epithelium on labia majora.
View Figure 1
Figure 1: VIN3; notice acetowhite epithelium on labia majora.
View Figure 1
A 48-year-old G8P6 HIV negative woman was seen at WHP clinic at Banso Baptist Hospital with a history of longstanding pruritus vulvae and vulvo-perineal discoloration. Similar to Case One, she had been followed for several months and treated presumptively for vulvar candidiasis, then a combination of antifungals and antibiotics, with no improvement. She was then referred to the WHP were her cervical cancer screening was negative. The vulva appeared highly hypopigmented and excoriated (Figure 2). Toluidine blue test (TBT) of the vulva was negative, but VIA revealed some faint acetowhite areas. Histopathology of biopsies from these acetowhite areas revealed IVC. She was treated by radical vulvectomy.
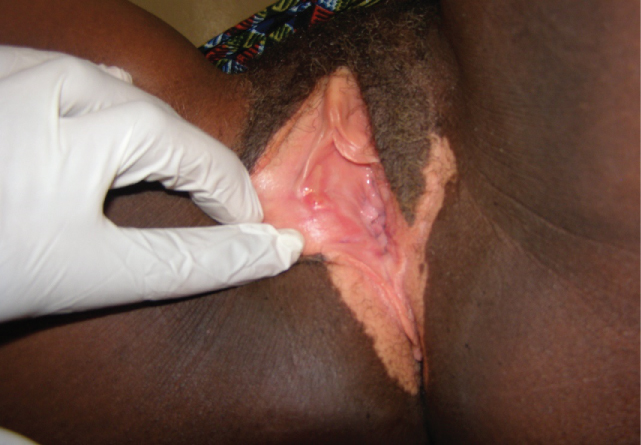 Figure 2: IVC characterized by vulvo-perineal discoloration.
View Figure 2
Figure 2: IVC characterized by vulvo-perineal discoloration.
View Figure 2
At Banso Baptist Hospital, a 33-year-old G3P2 woman living with HIV and on antiretroviral treatment was referred to the WHP clinic with a history of vulvar warts that had been treated with Podophyllum for several months to no avail. At WHP she complained of severe vulvar pain and difficulty walking, standing, sitting, and sleeping. On examination, the vulva had an extensive ulcerative mass with patches of necrosis mostly at the right labia majora extending to the symphysis pubis and the perineum (Figure 3). The lesion was firm, tender, and highly suspicious of IVC. It was impossible to perform cervical cancer screening due to inability to insert a vaginal speculum due to vulvo-vaginal tenderness and pressure from the vulvar mass. Vulvar histopathology confirmed an IVC.
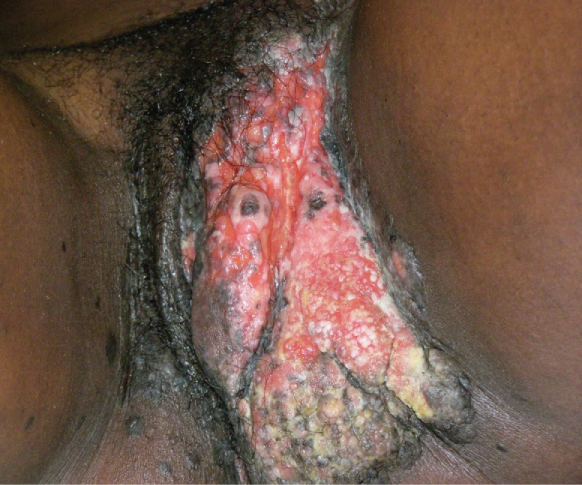 Figure 3: IVC.
View Figure 3
Figure 3: IVC.
View Figure 3
She was referred for radiotherapy at the only functional radiation oncology unit in Cameroon, at the Douala General Hospital but was unable to afford transport to Douala let alone the cost of treatment. She was then referred to the CBCHS Palliative Care Program for pain and symptom control.
A 35-year-old G6P5, HIV negative lady presented to WHP clinic at Baptist Hospital Mutengene for routine cervical cancer screening. She reported no symptoms at the time of screening. Cervical cancer screening was negative. As the speculum was being withdrawn from the vagina at the end of the screening, a whitish raised, non-tender leukoplakia of about 1 cm in diameter was noticed at the right labia minora (Figure 4). Punch biopsy was performed with a Tischler punch biopsy forceps under local anesthesia. Histopathology revealed IVC. When the result was communicated to her, she prayed with her pastor, who claimed that she had been cured by the prayers and that she did not need any more follow-up or treatment. Although WHP encouraged her to return for a reexamination to "confirm the healing", she refused.
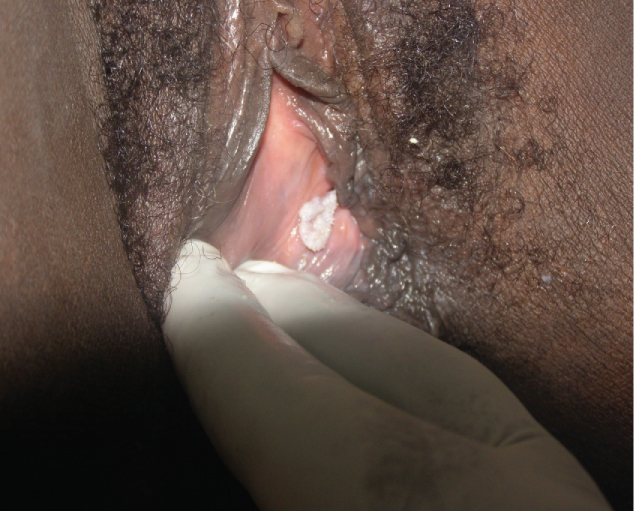 Figure 4: Leukoplakia containing IVC.
View Figure 4
Figure 4: Leukoplakia containing IVC.
View Figure 4
Disorders of the vulva have characteristically been given less attention than disorders of the cervix and other cancers, creating a challenge in diagnosis and treatment [14]. From these case studies, it is evident that the diagnosis of vulvar neoplasia is difficult, especially in LMICs. Cases are first mis-diagnosed and inappropriately treated during general outpatient consultation. Such patients are often not referred to specialized units until after several weeks or months of treatment failure. This leads to delay in getting appropriate treatment, with associated suffering, increased expense, progression of the disease, and poor prognosis. It is therefore important to educate general physicians and nurses on vulvar disorders such as vulvar neoplasia and to encourage them to refer the patients to specialized units earlier.
Although gynecologists and dermatologists are usually more familiar with conditions of the vulva including vulvar neoplasia [9], primary care clinicians experienced in cervical cancer screening and management of pre-invasive diseases may be better placed to diagnose vulvar neoplasia because they are often the first to see such patients in the course of screening for cervical and vaginal disorders [14].
In Cameroon as in many LMICs, specialties in dermatology and gynecology are limited. Thus, nurses skilled in cervical cancer screening and treatment of precancerous lesions, if adequately trained in the natural history of HPV-associated disorders, skilled in manipulating the lower genital tract, and biopsy, could play an indispensable role in the diagnosis of vulvar neoplasia. This diagnosis is primarily clinical, requiring thorough visual inspection of the vulva under good lighting conditions. The examination can be augmented with magnifying procedures like colposcopy [9], in combination with colorimetry, that is, the use of acetic acid and/or toluidine blue tests [15]. Since histopathology is the gold standard for diagnosing vulvar neoplasia [7], it is recommended that a biopsy be performed on every questionable or suspicious vulvar lesion.
Cases One and Two both presented with severe pruritus vulvae, a symptom that can mislead a care provider to initially consider common benign conditions such as vulvar candidiasis. Nevertheless, the provider needs to start thinking beyond vulvar candidiasis within a week or two of no response to antifungals. Case Two had vulvar discoloration in addition to intense pruritus vulvae; symptoms similar to lichen sclerosus [7]. It is not clear why she had vulvar discoloration. It could have been associated with the vulvar cancer alone, or she might have had concomitant lichen sclerosus, which was missed in the histopathology. The bottom line is every vulvar lesion characterized by discoloration and pruritus should be followed-up with a biopsy.
The TBT, used in both cases One and Two, yielded no benefit. TBT was widely used in the 80s and was thought to be very effective in diagnosing VIN [13,16]. In a retrospective study of women with histopathologically confirmed VIN, Joura and colleagues found TBT to be effective in diagnosing VIN 3 by 100% and VIN 1/2 by 83% (p < 0.001), a general sensitivity of 92% and a negative predictive value of 96% [13]. This sounds as if TBT is a perfect test for VIN. Although this study had statistically significant findings, their sample size was small (24 participants), and there was no control group for comparison. A 1971 study of 1071 asymptomatic women undergoing a combination of TBT and colposcopy suggests that toluidine blue is valuable in diagnosing vulvar neoplasia [15].
Contrary to the findings of these earlier studies on the value of TBT, a 2008 a literature review by Michelitti and colleagues on vulvar neoplasia diagnosis concluded that colorimetry (staining with acetic acid or toluidine blue) and magnification do not improve ability to diagnose vulvar neoplasia over naked eye inspection without staining [14]. Van Beurden and colleagues examined the vulvas of 40 asymptomatic women with 3-5% acetic acid and found that 30% developed acetowhite epithelium of the vulva, and 100% developed vestibular acetowhite changes [17]. They concluded that there is an over-reaction of acetic acid on the vulva which can cause misleading diagnosis. Another study combining TBT, colposcopy, and biopsy in 93 women found that TBT has a false negative of 85.8% in diagnosing VIN, false positive of 30% and false negative of 37.5% in both VIN and IVC [18].
Case Two was examined with acetic acid, and some faint ill-defined acetowhite change was present. With an already established IVC, one would have expected to see a huge, thick and raised acetowhite lesion, but this was not the case. Studies have found that the use of acetic acid on the vulva is non-specific, unreliable and mis-leading [9,19]. Therefore, VIA of the vulva is often not performed.
Case Three was treated with podophyllum for presumptive vulvar warts for several months before being referred to WHP. As is the case with many other cancer patients, she could not afford radiation therapy [20], so she was referred for palliative care, the only option for her.
Case Three was just 33 when she was diagnosed with vulvar cancer. She had suffered from intense pruritus vulvae for more than seven months before the clinical appearance of a lesion. Even though she had been consulting for pruritus vulvae, none of her providers thought of performing a vulvar biopsy. Perhaps a vulvar biopsy at those very early stages of her pruritus would have revealed a VIN, and she could have benefited from simple vulvectomy like Case One. Her positive HIV status probably placed her at a disadvantage of rapid disease progression, because HIV is a risk factor for HPV associated vulvar cancer [19]. Contrary to previous opinions that vulvar cancer is a disease of elderly or postmenopausal women, the disease is becoming more prevalent in younger women, especially in areas of high HIV prevalence [21,22].
Among the cases presented, Case Four was the only asymptomatic patient with a vulvar neoplasia manifested as a leukoplakia. In contrast to the other three cases, her lesion was located in an area that was difficult for her to see, at the inner aspect of the labia minora, and asymptomatic. This highlights the importance of suspecting every leukoplakia as a possible early neoplasm and performing a biopsy [23]. It also demonstrates the importance of carefully examining the labia and vaginal walls each time a woman is undergoing a speculum examination. This patient never returned for treatment nor follow up examination because her pastor had declared her healed. It would have been helpful if her pastor had been willing to refer her for reexamination to "confirm the healing". It is important for healthcare personnel to seek to work with traditional healers and faith healers to educate them about cancer and other diseases and try to persuade them to refer their patients for reexamination after the professed healing. This patient was especially vulnerable to accepting her pastor's declaration, because she was asymptomatic. Had she been having pain or pruritis, she would have been less likely to accept the pastor's declaration, unless she had had remission of symptoms.
Case Four is not unique in being misled by faith healers. WHP has had a number of women with cervical precancer, including some high-grade, whose spiritual leaders declared them healed and prevented them from returning to the clinic for treatment or reexamination. An incidental finding related to these cases and others is that a number of the women in the WHP who were diagnosed with cervical precancer and high-grade vulvar dysplasia, entertained seeing spiritual leaders to obtain healing. If their spiritual leaders declared they were healed, they did not return to the WHP and were not reexamined and/or treated. In such cases, cultural and spiritual practices that may bring peace of mind to these women, may serve as barriers to appropriate diagnosis and treatment, to the detriment of women's health in Cameroon.
The inability to pay for health services might be one of the reasons that pushes patients to seek faith-healing, which is usually free. Therefore, the poorer persons in the society, especially if they are asymptomatic, are often the most vulnerable to believe in faith-healers.
These case studies highlight the importance of taking vulvar complaints such as pruritus, discoloration and leukoplakia seriously. Every vulvar discoloration and leukoplakia should be followed-up with a biopsy. Women presenting with persistent non-candidal vulvar pruritus should be evaluated with biopsy. A major contributing factor to mis-diagnosis and mis-treatment of vulvar neoplasia is that many general clinical providers are not knowledgeable about vulvar neoplasia and its manifestations. It is important to educate clinicians on vulvar neoplasia to facilitate early diagnosis and referral.
The role of TBT in modern practice should be further studied. Finally, during every pelvic examination, providers should carefully examine the vulva, labia and vaginal walls, even in asymptomatic patients, to rule-out abnormalities that might indicate neoplasia.