Obstetrics and Gynaecology Cases - Reviews
Aggressive Complete Hydatidiform Mole Coexistent With A Normal Fetus During Pregnancy: Is There A Correlation Between Outcome, and Serum HCG Levels? A Report on 2 Cases and Review of the Literature
Nathalie Renard1,2*, SWA Nij Bijvank2, JWB de Groot3, RHM Verheijen4, HH De Haan2, AJ Kruse5 and J van Eyck2
1Department of obstetrics and gynaecology, University Hospital Ghent, Belgium
2Department of obstetrics and gynaecology, Isala Clinic Zwolle, The Netherlands
3Department of oncology, Isala Clinic Zwolle, The Netherlands
4Department of gynaecologic oncology, UMC Utrecht Cancer Center, University Medical Center Utrecht, The Netherlands
5Department of gynaecologic oncology, Maastricht University Medical Center, The Netherlands
*Corresponding author: Nathalie Renard, Department of obstetrics and gynaecology, University Hospital Ghent De Pintelaan 185, 9000 Ghent, Belgium, Tel: +32499164439, E-mail: nathalie_renard@hotmail.com
Obstet Gynecol Cases Rev, OGCR-3-089, (Volume 3, Issue 3), Case Series; ISSN: 2377-9004
Received: February 24, 2016 | Accepted: May 20, 2016 | Published: June 01, 2016
Citation: Renard N, Nij Bijvank SWA, de Groot JWB, Verheijen RHM, De Haan HH, et al. (2016) Aggressive Complete Hydatidiform Mole Coexistent With A Normal Fetus During Pregnancy: Is There A Correlation Between Outcome, and Serum HCG Levels? A Report on 2 Cases and Review of the Literature. Obstet Gynecol Cases Rev 3:089. 10.23937/2377-9004/1410089
Copyright: © 2016 Renard N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Two cases of complete hydatidiform mole with coexisting fetus are described. In the first case, the mole of this twin pregnancy displayed an aggressive behavior with early maternal onset of severe pre-eclampsia and lung and liver metastases. The second patient opted for termination of pregnancy prior to a possible onset of complications. Existing literature on this topic is also reviewed, in particular on the role of serum hCG levels to predict a favourable fetal prognosis.
Importance: Because the combination of a viable fetus with a hydatidifom mole is a rare clinical entity, no specific guidelines are in place to help clinicians and patients confronted with this type of pregnancy.
Objective: We try to define a parameter which may be useful in counseling patients whether or not to continue their pregnancy.
Evidence acquisition: A literature search was performed in Pubmed using the search terms 'hydatidiform mole' AND 'twin' AND 'pre-eclampsia' OR 'chemotherapy'.
Results: We found a correlation between rising serum HCG in the second trimester, and fetal demise. This information can be taken into account when counseling a patient with complete hydatidiform mole with coexisting fetus as to whether or not to continue the pregnancy.
Relevance: For clinicians and patients confronted with a complete hydatidiform mole with coexistent fetus, the trend in serum HCG in the second trimester could be of great use in decision-making.
Keywords
Complete hydatidiform mole, Twin, Pre-eclampsia, Chemotherapy
Introduction
Complete hydatidiform mole coexisting with a normal fetus (CHMCF) occurs in 1/22000-1/100000 pregnancies [1]. This rare entity poses several diagnostic and management challenges. First of all, it is important to distinguish CHMCF from partial mole. CHMCF comprises two separate products of conception: a normal and theoretically viable fetus, and a complete mole. In a partial mole, molar changes occur in the placenta of a non-viable, mostly (but not always) [2] triploid fetus, that is often structurally abnormal. Due to the non-viable nature of the fetal component, a partial mole will always be evacuated after it has been diagnosed, but the management of CHMCF is not so clear-cut. A CHMCF poses a mother-versus-fetus dilemma, where the risks for the mother (excessive bleeding, pre-eclampsia, hyperthyroidism, and persistent gestational trophoblastic disease) have to be outweighed against the chance of a live birth. Previous case reports and case series have found an estimate chance of live birth of 40% [3], however all authors stress the importance of individualizing management, taking into account prognostic factors such as the development of pre-eclampsia, or massive bleeding.
In this paper, we describe two cases of complete hydatidiform mole with a coexisting fetus. In one case the molar component displayed an aggressive behaviour with lung and liver metastases and severe pre-eclampsia, in the other the patient opted for early termination of pregnancy. We also reviewed the existing literature on this topic, with particular interest in serum hCG rate of decrease as a predictor for good fetal prognosis.
Case 1
A 33-year-old G3P2 woman presented at 14+5 weeks gestation with a complaint of ongoing vaginal blood loss and spotting during this pregnancy. Previous ultrasounds performed by her local midwife were reported to be normal. It was a spontaneous pregnancy.
Her obstetrical history was uneventful with two previous spontaneous deliveries at term. Her medical history comprised a depression, treated several years earlier. Ultrasound evaluation revealed a normal fetus consistent with 15 weeks gestation. A vaginal culture appeared negative for pathogens, and a Pap-test did not show dysplasia.
Because the spotting did not subside, she consulted again at 16 weeks gestational age. At that time, a deviant aspect of the placenta was noted, with a multicystic, vesicular component, and the suspicion of trophoblastic disease arose.
Serum hCG (Cobas, Roche diagnostics, Mannheim, Germany) at that time was 593.000 mIU/ml (peak level in normal pregnancies: 100000 mIU/ml at 10th week of gestation) [4].
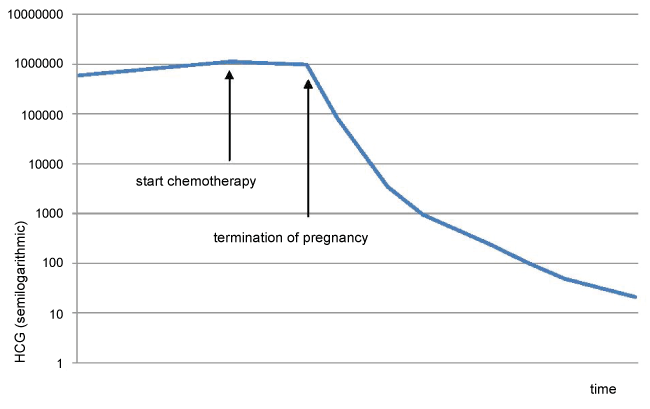
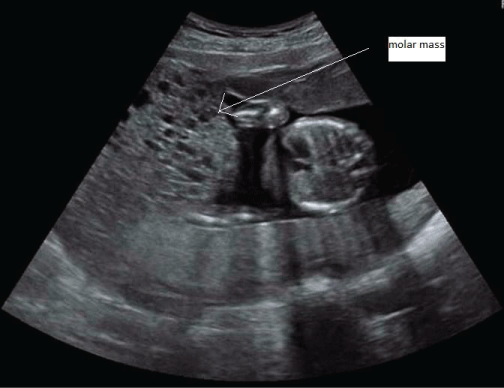
Further ultrasound confirmed the molar changes (Figure 1). The molar component continued to grow, the serum hCG rose, and fundal height was too high for gestational age. Thyroid function tests were repeatedly performed and remained normal. At 19 weeks gestational age, the coexistent fetus did not show structural abnormalities or growth restriction at ultrasonography. An amniocentesis was performed to exclude lethal chromosomal abnormalities, and revealed a normal fetal karyotype (46, XY). All these findings strongly suggested that this pregnancy was indeed a twin pregnancy comprising a complete hydatidiform mole and a normal coexisting fetus. The patient and her partner were counseled regarding risks and outcome of this type of pregnancy and chose to continue the pregnancy. She remained under close surveillance in our center. Serum hCG was repeatedly measured, and continued to rise (Figure 2).
.
Figure 1: Ultrasound image of patient 1 at gestational age 19 weeks, showing fetal thorax next to molar mass.
View Figure 1
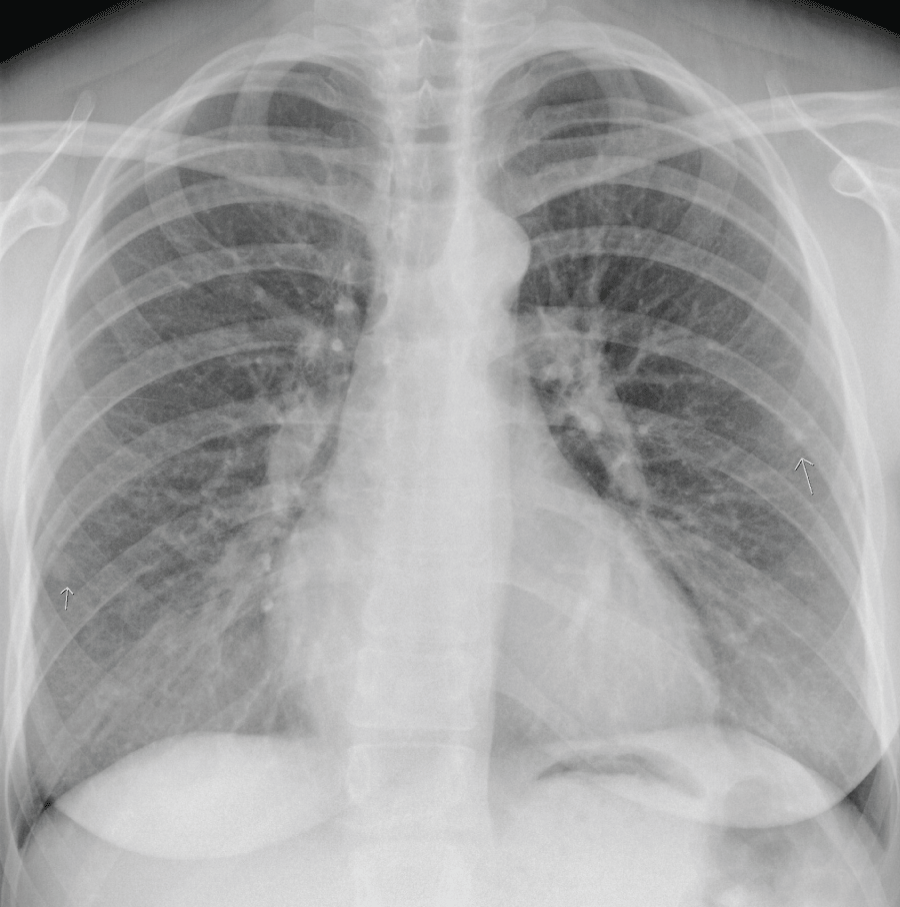
However, at 21+4 weeks gestation, the mother became increasingly dyspnoeic. A chest X-ray suggested multiple lung metastases (Figure 3). This was confirmed on a CT-scan of the thorax, that was performed to differentiate the lesions shown on chest X-ray from benign granulomas. A CT-scan of the brain revealed no metastatic lesions, a CT of the abdomen showed one lesion in the liver suggestive for a mestastasis. Serum hCG by that time had risen to 1.060.000 mIU/ml (Figure 2). According to the FIGO classification, she had a high risk Gestational Trophoblastic Neoplasia (GTN) stage IV, with a WHO prognostic score of 14 [5].
At this point, the couple was counseled about the recommendation to terminate the pregnancy and to start multi-agent chemotherapy [6], but they sincerely wished to continue the pregnancy until a viable newborn could be delivered.
The patient started polychemotherapy, with Bleomycine, Etoposide, Cisplatinum (BEP) since the more commonly used Etoposide, Methotrexate, Actinomycin D, Cyclophosphamide and Vincristine - regimen (EMA/CO-regimen) would be harmful for the coexistent fetus. However, before clinical response could be evaluated, one week following the start of chemotherapy, she developed severe pre-eclampsia with HELLP-syndrome at 22 weeks and 6 days of gestational age, necessitating immediate termination of pregnancy.
Since the risk of massive intrapartum hemorrhage seemed high, the patient decided to have a primary hysterectomy after counselling.
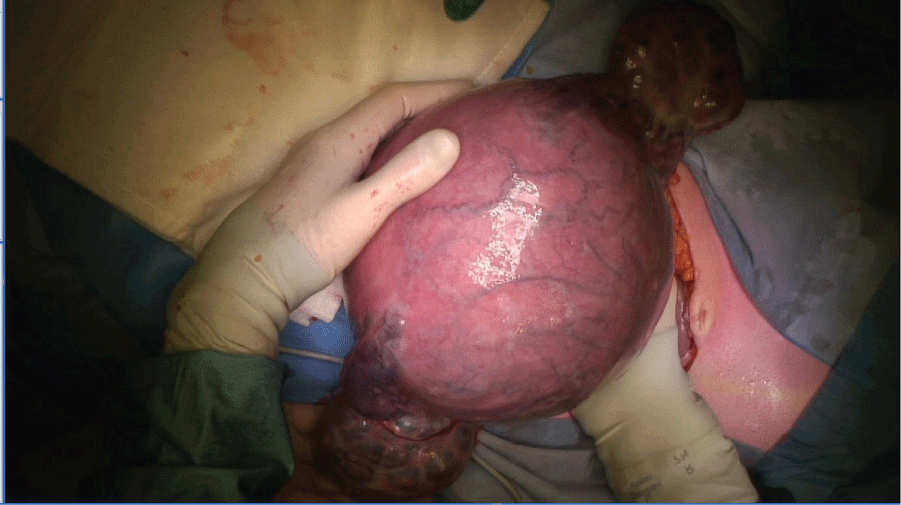
Vascular sheets were placed in the internal iliac artery bilaterally prior to the procedure, and inflated during surgery. Under general anesthesia, a hysterectomy was performed where both fetus and mole were left in utero. Both ovaries were significantly enlarged with theca lutein cysts and also bled easily during manipulation, and thus needed to be subsequently removed (Figure 4). A male fetus of 570 grams was delivered from the excised uterus, with no apparent structural abnormalities, who died postpartum due to immaturity.
After surgery, the patient was switched to the EMA/CO chemotherapy regimen [6,7], with clinical improvement. HCG levels fell steadily and returned to normal after 4 cycles of EMA/CO. After 7 cycles of EMA/CO a CT-scan showed a complete remission. One year after completion of treatment there is no evidence of disease recurrence. Pathologic investigation with p57 staining of the hysterectomy specimen confirmed the diagnosis of invasive complete mole (see figure 5 for a macroscopic image of the complete mole).

.
Figure 5: Pathology photograph of the placenta, showing the typical vesicular, grape-like lesions of a hydatidiform mole.
View Figure 5
Case 2
A 38-year old G8P7 patient was referred to our center for suspicion of a molar pregnancy on ultrasound investigation. She was 13+4 weeks along her 8th pregnancy, and experienced no bothersome symptoms other than some minimal vaginal blood loss. Her previous medical and surgical history was uneventful. The pregnancy was spontaneous. Prior ultrasound investigations were suggestive for a vanishing twin. In our center, ultrasound investigation demonstrated a vital intrauterine singleton in combination with a molar pregnancy (Figure 6).
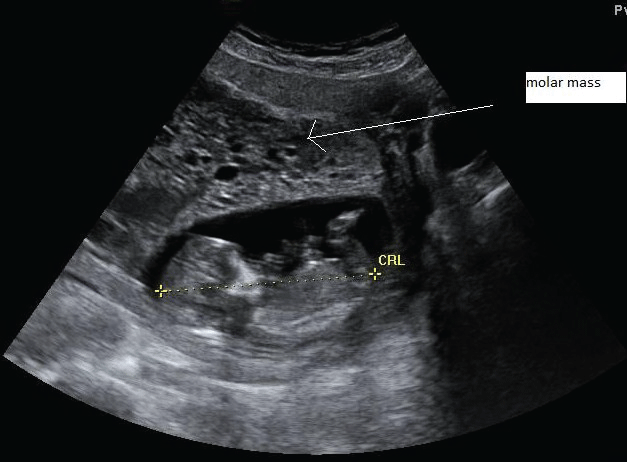
.
Figure 6: Ultrasound image of patient 2 during the 14th week of gestation, showing a structurally normal fetus alongside a molar mass.
View Figure 6
Serum HCG level at the time of admission was 611.000 mIU/ml. Her lab results showed hyperthyroidism, treatment was started with Thiamazol and Propranolol.
Her chest X-ray was normal, and an ultrasound scan and MRI of the liver showed no metastatic lesions. Given the very suggestive sonographic findings, biopsy was not performed.
The patient and her partner were counseled about the different options, and chose termination of pregnancy by means of abdominal hysterectomy.
Pathologic examination of the hysterectomy specimen confirmed a complete, non-invasive mole, with a concomitant normal fetus without any gross pathological abnormalities.
Postoperative hCG-levels fell rapidly. HCG two weeks post-operatively was 821 mIU/mL, 6 weeks postoperatively 4.1 mIU/ml. Hyperthyroidism subsided.
Literature Search, Materials and Methods
A literature search was performed in Pubmed using the search terms 'hydatidiform mole' AND 'twin' AND 'pre-eclampsia' OR 'chemotherapy'. This led to 55 eligible articles. Of this list, papers were selected on the basis of title and abstract. Reference lists of selected articles were searched manually to retrieve additional articles that might be of interest to our current study. The distinction between complete and partial mole was made only in the year 1977 [8,9]. Therefore, we only considered literature from 1977, until March 2016.
Statistical analysis was performed using JMP 12 (SAS Institute Inc., Cary, NC, USA). A Fisher's exact test was performed to analyze the correlation between hCG-trend in the second trimester, and fetal outcome.
Discussion and Review of the Literature
Although CHMCF remains a rare entity, with a cited incidence of 1/22000-1/100000 pregnancies [1], we found numerous case reports and case series describing this exceptional type of twin pregnancy. However, due to the small numbers, management guidelines are not yet defined. Treatment is suggested to be individualized for each single patient.
Before counseling the patient, a definite diagnosis of CHMCF is needed. A clinical suspicion of CHMCF may arise due to ultrasound findings, namely a cystic multilocular component of the placenta. Differential diagnosis of these findings include partial hydatidiform mole, molar degeneration of a normal placenta following spontaneous abortion [10], placental hematomas, focal hydropic placental changes, chorioangioma [11], mesenchymal dysplasia [2] or even a partially necrotic leiomyoma [12].
While the latter entities may be very rare, it is important to distinguish the former, namely partial hydatidiform mole, from CHMCF, due to the non-viable nature of the fetus in partial mole. When suspecting CHMCF, an amniocentesis should be performed to ensure a normal fetal karyotype (although non-triploid partial moles and confined mosaicism are well known pitfalls [2]). Besides, structural ultrasound is indicated to exclude fetal abnormalities, and if possible cytogenetic or immunohistochemical analysis of the molar component is performed to confirm its androgenetic origin [13-15]. If the pregnancy is indeed found to be a partial hydatidiform mole, termination of pregnancy (TOP) and evacuation of the mole should be advised. However, if by these investigations the diagnosis of CHMCF is confirmed, the patient should be counseled about the risks of continuing the pregnancy versus the chance of delivering a healthy neonate.
Large case series [3,11,16-18] have found an estimated chance of 21% [18] to 40% [3] of delivering a live infant. This chance may possibly be underestimated, because a significant portion of CHMCF pregnancies may be terminated early upon diagnosis, but might have led to the birth of a live neonate if continued.
Several authors [11,19,20] suggest that when less aggressive trophoblast is noted (i.e. decreasing size of the molar component, falling serum hCG-levels in the second trimester, uterine size that is not abnormally large for dates) chances for a successful outcome of the pregnancy, are increased.
In this perspective, Marcorelles et al. [19] suggest two types of CHMCF pregnancy: one in which the molar part becomes quiescent and even necrotic, with successful outcome, and one where the molar part grows extensively, leading to severe maternal complications and often fetal demise.
If this theory would be true, prediction of a favourable or non-favourable outcome might be provided by hCG course. We therefore decided to investigate the trend in serum hCG-levels in relation to fetal outcome. So far, we found a total of 91 live babies born after a CHMCF pregnancy described in the literature (see Table 1 for reviewed articles [2,3,10,11,16-55]). The trend in serum hCG-levels (rather than absolute numbers, since not all authors employ the same measurement units and/or measurement assays) could be correlated with fetal outcome (Table 2).
![]()
Table 1: Reviewed articles of CHMCF pregnancies.
View Table 1
![]()
Table 2: Correlation of trend in serum HCG, and fetal outcome.
View Table 2
This correlation was analyzed by a Fisher's exact test, resulting in a p-value of 0.0007, indicating that there is indeed a correlation between hCG-trend in the second trimester, and fetal outcome.
Limitations of our study are: a small sample size, a binary rather than continuous exposure variable, and a non-systematic literature review.
More questions need to be answered before this correlation can be translated into a definite, clear-cut guideline: at what gestational age exactly should serum hCG-levels start to decrease? Can a cutoff value be defined of hCG-level at a certain gestational age?
The chance of live birth must be outweighed against the risks for the mother, notably the development of severe bleeding, hyperthyroidism and thyroid crisis, pre-eclampsia, and post-molar Gestational Trophoblastic Neoplasia (GTN).
Previous case reports and case series have found a risk of GTN of 19-57% [3,16,17,28], which is more than after a singleton molar pregnancy.
Although the risk of developing GTN is reported to be higher in CHMCF than in singleton mole pregnancy in some series (18-57%) [11,21], the largest case series reported by Sebire et al. [3] found that the risk of GTN was equal to that in singleton moles.
Interestingly, the risk of developing GTN does not seem to increase with increasing gestational age [17]. This warrants a conservative approach in CHMCF pregnancy if there are no other indications for immediate termination. Conversely, this also means that a rigorous follow-up should be pursued also in those cases that chose early TOP.
Also a higher incidence of severe maternal complications is observed in patients who subsequently developed GTN [17]. The risk of developing GTN and other maternal complications such as pre-eclampsia is theoretically higher in those CHMCF pregnancies with rising serum hCG in the second trimester [2,33].
Absolute indications for TOP are: thyroid crisis, evidence of trophoblastic metastases, pre-eclampsia, life-threatening vaginal bleeding, and severe hyperemesis gravidarum [11,33].
Relative indications for TOP are a fundal height that is too high for dates [24], and a serum hCG that does not fall in the second trimester [11,42,44,45] .
If the patient chooses to continue CHMCF pregnancy, a thorough search for metastatic disease should be initiated and repeated serially [26], and she should be monitored closely for signs and symptoms of hyperthyroidism, pre-eclampsia, vaginal bleeding, excessive molar growth and uterine expansion, and trophoblastic metastases.
Ultrasound and serum hCG-levels should be checked on a regular basis [36].
The patient described by us in the first case developed pulmonary and liver metastases at 21 weeks gestational age, indicating indeed the aggressive nature of this mole. She was very adamant about her wish to continue the pregnancy, thus when the need for chemotherapy arose with the occurrence of metastatic disease, she was started on BEP since the more commonly used EMA/CO-regimen is teratogenic.
Despite the fact that she started with chemotherapy, she developed severe pre-eclampsia and HELLP-syndrome, and TOP could no longer be postponed.
After TOP, the signs and symptoms of the pre-eclampsia subsided and serum hCG-levels started to fall. Subsequently, chemotherapy was switched to EMA/CO, according to management guidelines [6], resulting in complete biochemical and radiological remission.
In retrospect, the rising serum hCG-level in the second trimester might have been indicative of poor fetal prognosis, and she could have been counseled towards earlier TOP with fewer consequences.
Conclusion
We found a correlation between serum hCG trend in the second trimester, and fetal outcome, albeit based on a small number of observations. We would like to encourage future authors of CHMCF case reporting to adjoin serum hCG trends in their articles, to add to the small body of evidence of this observation and to be able to formulate definite management guidelines for CHMCF pregnancies. The first case report also demonstrates several ominous findings that were associated with fetal demise, namely a fundal height that is too large for gestational age, an ongoing growth of the molar component and the development of theca lutein cysts and pre-eclampsia.
These two cases once again underline the difficult mother-versus-fetus dilemma that arises in CHMCF pregnancy, and tries to deliver suggestions for management based on previous experiences described in the literature for patients and clinicians who are confronted with this very rare type of pregnancy.
Contribution to Authorship
Nathalie Renard: Conception and writing of the article, literature search, acquisition of data, analysis and interpretation of data.
Nij Bijvank, de Groot, De Haan and Verheijen: Revision of the article.
Van Eyck: Revision of the article, and final approval of the version to be published.
Details of Ethics Approval
Both our patient have given approval for publication of this article with the images included.
References
-
Jones WB, Lauersen NH (1975) Hydatidiform mole with coexistent fetus. Am J Obstet Gynecol 122: 267-272.
-
Jauniaux E, Bersinger NA, Gulbis B, Meuris S (1999) The contribution of maternal serum markers in the early prenatal diagnosis of molar pregnancies. Hum Reprod 14: 842-846.
-
Sebire NJ, Foskett M, Paradinas, Fisher RA, Francis RJ FJ, et al. (2002) Outcome of twin pregnancies with complete hydatidiform mole and healthy co-twin. Lancet 359: 2165-2166.
-
Cunningham FH, Hauth CH, Leveno KJ, Gilstrap L, Bloom SL, et al. (2005) Williams Obstetrics, 22nd edition, McGraw-Hill, USA.
-
FIGO Oncology Committee (2002) FIGO staging for gestational trophoblastic neoplasia 2000. Int J Gynaecol Obstet 77: 285-287.
-
Horowitz NS, Goldstein DP, Berkowitz RS (2009) Management of gestational trophoblastic neoplasia. Semin Oncol 36: 181-189.
-
Bower M, Newlands ES, Holden L, Short D, Brock C, et al. (1997) EMA/CO for high-risk gestational trophoblastic tumors: results from a cohort of 272 patients. J Clin Oncol 15: 2636-2643.
-
Vassilakos P, Riotton G, Kajii T (1977) Hydatidiform mole: two entities. A morphologic and cytogenetic study with some clinical consideration. Am J Obstet Gynecol 127: 167-170.
-
Kajii T, Ohama K (1977) Androgenetic origin of hydatidiform mole. Nature 268: 633-634.
-
Miller D, Jackson R, Ehlen T, McMurtrie E (1993) Complete hydatidiform mole coexistent with a twin live fetus: clinical course of four cases with complete cytogenetic analysis. Gynecol Oncol 50: 119-123.
-
Bristow RE, Shumway JB, Khouzami AN, Witter FR (1996) Complete hydatidiform mole and surviving coexistent twin. Obstet Gynecol Surv 51: 705-709.
-
Sauerbrei EE, Salem S, Fayle B (1980) Coexistent hydatidiform mole and live fetus in the second trimester: an ultrasound study. Radiology 135: 415-417.
-
Ishii J, Iitsuka Y, Takano H, Matsui H, Osada H, et al. (1998) Genetic differentiation of complete hydatidiform moles coexisting with normal fetuses by short tandem repeat-derived deoxyribonucleic acid polymorphism analysis. Am J Obstet Gynecol 179: 628-634.
-
Castrillon DH, Sun D, Weremowicz S, Fisher RA, Crum CP, et al. (2001) Discrimination of complete hydatidiform mole from its mimics by immunohistochemistry of the paternally imprinted gene product p57KIP2. Am J Surg Pathol 25: 1225-1230.
-
Fisher RA, Tommasi A, Short D, Kaur B, Seckl MJ, et al. (2014) Clinical utility of selective molecular genotyping for diagnosis of partial hydatidiform mole; a retrospective study from a regional trophoblastic disease unit. J Clin Pathol 67: 980-984.
-
Fishman DA, Padilla LA, Keh P, Cohen L, Frederiksen M, et al. (1998) Management of twin pregnancies consisting of a complete hydatidiform mole and normal fetus. Obstet Gynecol 91: 546-550.
-
Matsui H, Sekiya S, Hando T, Wake N, Tomoda Y (2000) Hydatidiform mole coexistent with a twin live fetus: a national collaborative study in Japan. Hum Reprod 15: 608-611.
-
Massardier J, Golfier F, Journet D, Frappart L, Zalaquett M, et al. (2009) Twin pregnancy with complete hydatidiform mole and coexistent fetus: obstetrical and oncological outcomes in a series of 14 cases. Eur J Obstet Gynecol Reprod Biol 143: 84-87.
-
Marcorelles P, Audrezet MP, Le Bris MJ, Laurent Y, Chaboud JJ, et al. (2005) Diagnosis and outcome of complete hydatidiform mole coexisting with a live twin fetus. Eur J Obstet Gynecol Reprod Biol 118: 21-27.
-
Piura B, Rabinovich A, Hershkovitz R, Maor E, Mazor M (2008) Twin pregnancy with a complete hydatidiform mole and surviving co-existent fetus. Arch Gynecol Obstet 278: 377-382.
-
Steller MA, Genest DR, Bernstein MR, Lage JM, Goldstein DP, et al. (1994) Natural history of twin pregnancy with complete hydatidiform mole and coexisting fetus. Obstet Gynecol 83: 35-42.
-
Anderson CK, Deiter RW, Motz MJ, Goldstein JA (1996) Complete hydatidiform mole with a coexistent healthy, viable fetus near term: a case report. J Reprod Med 41: 55-58.
-
Hurteau JA, Roth LM, Schilder JM, Sumners J (1997) Complete hydatidiform mole coexisting with a twin live fetus: clinical course. Gynecol Oncol 66: 156-159.
-
Shahabi S, Naome G, Cobin L, Verougstraete A, Masters L, et al. (1997) Complete hydatidiform mole and coexisting normal fetuses. A report of two cases with contrasting outcomes. J Reprod Med 42: 756-760.
-
Chen FP (1997) Molar pregnancy and living normal fetus coexisting until term: prenatal biochemical and sonographic diagnosis. Hum Reprod 12: 853-856.
-
Kauffman DE, Sutkin G, Heine RP, Watt-Morse M, Price FV (1999) Metastatic complete hydatidiform mole with a surviving coexistent twin. A case report. J Reprod Med 44: 131-134.
-
Abbi M, Kriplani A, Uppal R, Takkar D (1999) Term twin pregnancy with hydatidiform mole and a normal fetus. Arch Gynecol Obstet 262: 189-191.
-
Bruchim I, Kidron D, Amiel A, Altaras M, Fejgin MD (2000) Complete hydatidiform mole and a coexistent viable fetus: report of two cases and review of the literature. Gynecol Oncol 77: 197-202.
-
Albers E, Daneshmand S, Hull A (2001) Placental pathology casebook. Complete hydatidiform mole with coexistent term twin pregnancy. J Perinatol 21: 72-75.
-
Kwon HE, Park EJ, Kim SH, Chae HD, Won HS, et al. (2002) A case of twin pregnancy with complete hydatidiform mole and coexisting fetus following IVF-ET. J Assist Reprod Genet 19: 144-148.
-
Moini A, Riazi K (2003) Molar pregnancy with a coexisting fetus progressing to a viable infant. Int J Gynaecol Obstet 82: 63-64.
-
Aggarwal N, Suri V, Deo ND, Malhotra S, Vasishta K (2004) Twin pregnancy with a complete hydatidiform mole and co-existing fetus. Acta Obstet Gynecol Scand 83: 604.
-
Steigrad SJ, Robertson G, Kaye AL (2004) Serial hCG and ultrasound measurements for predicting malignant potential in multiple pregnancies associated with complete hydatidiform mole: a report of 2 cases. J Reprod Med 49: 554-558.
-
Lambert-Messerlian G, Pinar H, Rubin LP, De Paepe ME, Tantravahi U, et al. (2005) Second-trimester maternal serum markers in twin pregnancy with complete mole: report of 2 cases. Pediatr Dev Pathol 8: 230-234.
-
Vaisbuch E, Ben-Arie A, Dgani R, Perlman S, Sokolovsky N, et al. (2005) Twin pregnancy consisting of a complete hydatidiform mole and co-existent fetus: report of two cases and review of literature. Gynecol Oncol 98: 19-23.
-
Wee L, Jauniaux E (2005) Prenatal diagnosis and management of twin pregnancies complicated by a co-existing molar pregnancy. Prenat Diagn 25: 772-776.
-
Hancock BW, Martin K, Evans CA, Everard JE, Wells M (2006) Twin mole and viable fetus: The case for misdiagnosis. J Reprod Med 51: 825-828.
-
Ogura T, Katoh H, Satoh S, Tsukimori K, Hirakawa T, et al. (2006) Complete mole coexistent with a twin fetus. J Obstet Gynaecol Res 32: 593-601.
-
Niemann I, Sunde L, Petersen LK (2007) Evaluation of the risk of persistent trophoblastic disease after twin pregnancy with diploid hydatidiform mole and coexisting normal fetus. Am J Obstet Gynecol 197: 45.
-
True DK, Thomsett M, Liley H, Chitturi S, Cincotta R, et al. (2007) Twin pregnancy with a coexisting hydatiform mole and liveborn infant: complicated by maternal hyperthyroidism and neonatal hypothyroidism. J Paediatr Child Health 43: 646-648.
-
Yamada T, Matsuda T, Kudo M, Yamada T, Moriwaki M, et al. (2008) Complete hydatidiform mole with coexisting dichorionic diamniotic twins following testicular sperm extraction and intracytoplasmic sperm injection. J Obstet Gynaecol Res 34: 121-124.
-
Dolapcioglu K, Gungoren A, Hakverdi S, Hakverdi AU, Egilmez E (2009) Twin pregnancy with a complete hydatidiform mole and co-existent live fetus: two case reports and review of the literature. Arch Gynecol Obstet 279: 431-436.
-
Albayrak M, Ozer A, Demir OF, Ozer S, Erkaya S (2010) Complete mole coexistent with a twin fetus. Arch Gynecol Obstet 281: 119-122.
-
Lee SW, Kim MY, Chung JH, Yang JH, Lee YH, et al. (2010) Clinical findings of multiple pregnancy with a complete hydatidiform mole and coexisting fetus. J Ultrasound Med 29: 271-280.
-
Kihara M, Usui H, Tanaka H, Inoue H, Matsui H, et al. (2012) Complicating preeclampsia as a predictor of poor survival of the fetus in complete hydatidiform mole coexistent with twin fetus. J Reprod Med 57: 325-328.
-
Dalmia S, Sivakumar K, Kathirvel R, Phillips K, Coady AM (2013) Twin pregnancy with a complete hydatidiform mole and co-existent healthy fetus: unusual case of complete resorption of molar pregnancy. J Obstet Gynaecol 33: 312-313.
-
Vimercati A, de Gennaro AC, Cobuzzi I, Grasso S, Abruzzese M, et al. (2013) Two cases of complete hydatidiform mole and coexistent live fetus. J Prenat Med 7: 1-4.
-
Ferraz TJ, Bartosch CM, Ramalho CM, Carvalho FA, Carvalho BC, et al. (2013) Complete mole in a dichorionic twin pregnancy after intracytoplasmic sperm injection. Rev Bras Ginecol Obstet 35: 39-43.
-
Huang X, Liang J, Huang Y, Huang J (2014) Complete hydatidiform mole and a coexistent fetus following ovulation induction in a patient with Sheehan's syndrome: a first case report and review of literature. Arch Gynecol Obstet 289: 1145-1150.
-
Okumura M, Fushida K, Francisco RP, Schultz R, Zugaib M (2014) Massive necrosis of a complete hydatidiform mole in a twin pregnancy with a surviving coexistent fetus. J Ultrasound Med 33: 177-179.
-
Kutuk MS, Ozgun MT, Dolanbay M, Batukan C, Uludag S, et al. (2014) Sonographic findings and perinatal outcome of multiple pregnancies associating a complete hydatiform mole and a live fetus: a case series. J Clin Ultrasound 42: 465-471.
-
Peng HH, Huang KG, Chueh HY, Adlan AS, Chang SD, et al. (2014) Term delivery of a complete hydatidiform mole with a coexisting living fetus followed by successful treatment of maternal metastatic gestational trophoblastic disease. Taiwan J Obstet Gynecol 53: 397-400.
-
Buke B, Topcu HO, Bulgu E, Eminov E, Kazandi M (2014) Complete hydatidiform mole presenting as placenta previa in a twin pregnancy with a coexisting normal foetus: Case report. J Turk Ger Gynecol Assoc 15: 256-258.
-
Rohilla M, Singh P, Kaur J, Jain V, Gupta N, et al. (2015) Individualistic approach to the management of complete hydatidiform mole with coexisting live fetus. Eur J Obstet Gynecol Reprod Biol 191: 39-42.
-
Sheik S, Al-Riyami N, Mathew NR, Al-Sukaiti R, Qureshi A, et al. (2015) Twin Pregnancy with a Complete Hydatidiform Mole and a Coexisting Live Fetus: Rare entity. Sultan Qaboos Univ Med J 15: e550-e553.