Obstetrics and Gynaecology Cases - Reviews
An Idiopathic Case of Recurrent Spontaneous Ovarian Hyper Stimulation Syndrome
Alessandra Ainsworth*, Zaraq Khan and Jani Jensen
Mayo Clinic School of Graduate Medical Education, Division of Reproductive Endocrinology, MN, USA
*Corresponding author: Alessandra Ainsworth, Mayo Clinic School of Graduate Medical Education, Division of Reproductive Endocrinology, 200 First Street SW, Rochester MN 55905, USA, Tel: (507) 266-3262, Fax: (507) 284-9684, E-mail: ainsworth.alessandra@mayo.edu
Obstet Gynecol Cases Rev, OGCR-3-081, (Volume 3, Issue 2), Case Report; ISSN: 2377-9004
Received: December 20, 2015 | Accepted: March 23, 2016 | Published: March 26, 2016
Citation: Ainsworth A, Khan Z, Jensen J (2016) An Idiopathic Case of Recurrent Spontaneous Ovarian Hyper Stimulation Syndrome. Obstet Gynecol Cases Rev 3:081. 10.23937/2377-9004/1410081
Copyright: © 2016 Alessandra A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
We report a case of recurrent spontaneous ovarian hyperstimulation syndrome (OHSS). The patient presented at 8 weeks gestation. Due to severity of symptoms, the patient required both inpatient and outpatient management. Her symptoms resolved at 11 weeks gestation and the pregnancy was carried to term. Recurrent spontaneous OHSS is a rare and potentially morbid event. While most cases will eventually resolve, patients require careful diagnosis, surveillance, and supportive care.
Keywords
Spontaneous Ovarian Hyperstimulation Syndrome, OHSS
Introduction
Ovarian hyperstimulation syndrome (OHSS) is almost exclusively a complication of assisted reproductive technology (ART), with a reported incidence of 2.3% per patient [1]. Increased risk of ART associated OHSS is found in patients of young age, low body weight, and polycystic ovary syndrome (PCOS) [2]. Rarely, OHSS has been reported following spontaneous conception [3,4]. Iatrogenic and spontaneous cases of OHSS are clinically alike. OHSS is characterized by significant ovarian enlargement, extravascular fluid shifts, and a spectrum of clinical outcomes ranging from mild nausea and vomiting to significant ascites, end organ hypoperfusion, and possible death. OHSS, regardless of etiology, will eventually resolve. However, resolution may take weeks and requires close surveillance and supportive therapy. We report a case of recurrent OHSS following spontaneous conception.
Case Report
A 29-year-old gravida 3, para 1-0-1-1, presented at 8 weeks gestation after spontaneous conception with progressive abdominal discomfort, nausea, and a three kilogram weight gain over the past week. She had established prenatal care at an outside institution prior to this visit. She had no personal history of thyroid disease, pituitary pathology, or polycystic ovary syndrome, and had never used medications for ovulation induction or undergone other fertility treatment. Her medical history was consistent with apparent spontaneous OHSS with her two prior pregnancies. There was no family history of spontaneous OHSS.
In her first pregnancy, early pregnancy loss was diagnosed at nine weeks and treated with dilation and curettage. Ultrasound images from an outside institution showed bilaterally enlarged ovaries containing multiple follicles and fluid within the abdomen, but OHSS was not considered or recognized. Her second pregnancy was complicated by late onset, severe OHSS. At approximately 7 weeks gestation, with a viable singleton intrauterine pregnancy, she developed significant ascites, pleural effusions, and concern for acute renal failure. She required admission to the intensive care unit (ICU) for supportive care and ultimately had resolution of symptoms around 10 weeks of gestation. The remainder of the pregnancy was uneventful and she delivered vaginally at 37 0/7 weeks. The patient was cared for at an outside institution for both of these pregnancies.
The patient was referred to our institution for preconception counseling after her second delivery. She was advised that in vitro fertilization may allow for a more controlled early pregnancy course, with use of embryo cryopreservation and either temporally delayed embryo transfer or use of a gestational carrier. She did not pursue this treatment and naturally conceived her third pregnancy.
Upon presentation with her third pregnancy at 8 weeks, she appeared uncomfortable but had stable vital signs with a blood pressure of 100/68. Pelvic ultrasound demonstrated a viable intrauterine pregnancy, bilaterally enlarged ovaries (9.7 × 9.0 × 11.9 cm and 13.5 × 8.8 × 9.9 cm), mild ascites, and a small pleural effusion. Electrolytes, coagulation profile, complete blood count, and liver function tests were all within normal limits. TSH was 3.9 mIU/L (normal range 0.3-5.0 mIU/L). She was diagnosed with spontaneous OHSS and started on cabergoline to decrease vascular permeability. Initially managed as an outpatient, the patient was assessed by daily phone calls to monitor vital signs, body weight, fluid intake and urine output, and abdominal circumference.
She reported increasing dyspnea and decreased urine output two days later and was admitted to the hospital for observation. On day of admission, repeat ultrasound revealed a viable singleton gestation, progressive bilateral ovarian enlargement (12.4 × 12.5 × 17.3 cm and 16.5 × 12.5 × 11.5 cm), moderate ascites, and moderate right pleural effusion. Laboratory evaluation revealed hemoconcentration (Hgb 17.7 g/dL) and hyponatremia (129 mmol/L). A therapeutic thoracentesis removed 300 cc of clear serosanguineous fluid. She was started on Heparin 5000 units BID and intravenous fluids while cabergoline was continued. The patient symptomatically improved on hospital day two. Repeat laboratory studies revealed decreasing hemoconcentration (Hgb 15.3 g/dL) and resolution of hyponatremia (132 mmol/L). A second thoracentesis removed 900cc of amber-colored fluid. She was discharged to home on hospital day two.
The patient was seen for follow-up the next day and complained of persistent dyspnea and progressive ovarian enlargement (19.7 × 14.7 × 12.9 cm and 18.2 × 13.2 × 13.1 cm) though pregnancy remained viable (Figure 1). She was followed closely as an outpatient, receiving five serial therapeutic thoracenteses. Her symptoms resolved thereafter, and the remainder of her pregnancy was uncomplicated. She was induced at 39 0/7 weeks and delivered a live born male via spontaneous vaginal delivery without complication. A postpartum tubal ligation was performed.
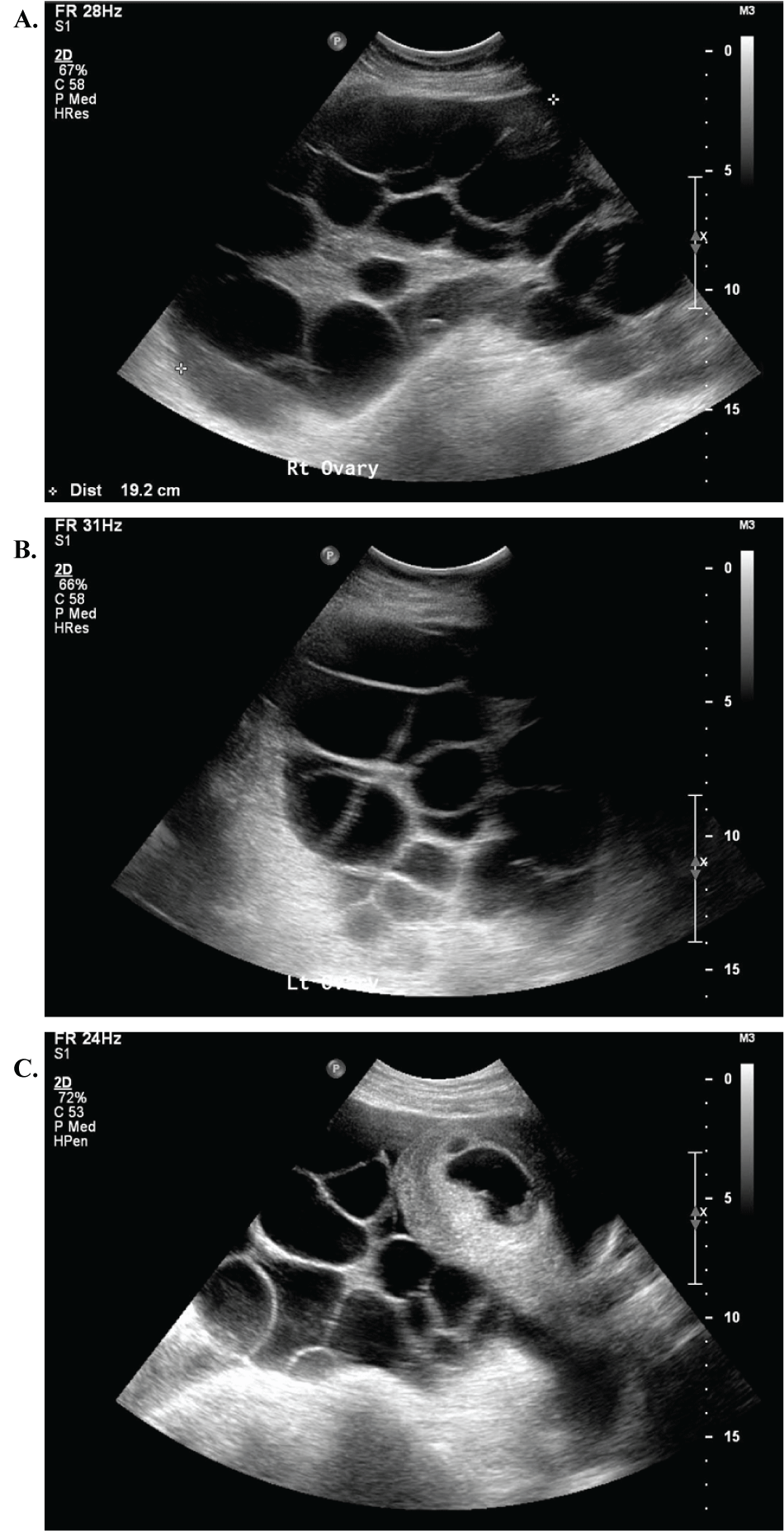
.
Figure 1: Bilaterally enlarged ovaries with multiple large follicles and viable singleton intrauterine pregnancy. (A) Right ovary measuring 19.7 × 14.7 × 12.9 cm; (B) Left ovary measuring 18.2 × 13.2 × 13.1 cm; (C) Intrauterine pregnancy with cardiac pulsations. Ovarian size can be appreciated in contrast with the size of the gravid uterus.
View Figure 1
Discussion
Spontaneous OHSS results from excessive stimulation of follicle stimulating hormone (FSH) receptors on ovarian granulosa cells. This stimulation leads to the recruitment of additional granulosa cells, local estrogen production, and eventual recruitment of multiple dominant follicles. Upon luteinization, these follicles release vasoactive substances, most notably vascular endothelial growth factor (VEGF), leading to systemic capillary leakage, extravascular fluid shifts, and intravascular hypovolemia [2]. Clinical outcomes range from mild patient discomfort to end-organ failure, coagulopathy risk and possible death.
The spectrum of OHSS has been classified based on signs, symptoms, and laboratory findings. Grade I OHSS is characterized by bilateral ovarian enlargement (≤ 5 × 5cm), serum estradiol greater than 1500 pg/mL, and progesterone concentration greater than 30 ng/mL. Grade II OHSS describes progressive ovarian enlargement (≤ 12 × 12 cm), abdominal discomfort, and gastrointestinal symptoms of nausea, vomiting, or diarrhea. Grade III OHSS is severe, and includes extreme ovarian enlargement (> 12 × 12 cm), ascites, pleural and/or pericardial effusion, electrolyte imbalances, hypovolemia, and hemoconcentration.
Patients with mild OHSS may be managed as an outpatient with daily communication of patient weight, abdominal circumference, and reported fluid intake and output. Serial laboratory evaluations for hematocrit, electrolytes, and creatinine should be reviewed for signs of disease progression. Hospitalization is required for serious disease. Indications for admission include uncontrolled pain, intractable nausea or vomiting, oliguria, dyspnea, electrolyte imbalances (hyponatremia: sodium < 135 mEq/L or hyperkalemia: potassium > 5 mEq/L), or hemoconcentration (Hct > 45%). Progression of symptoms, vital signs, and laboratory findings must be carefully monitored.
Supportive care with IV fluid hydration and ultrasound-guided paracentesis or thoracentesis may be needed to treat intravascular hypovolemia and extravascular volume overload. Anticoagulation with prophylactic Heparin or low molecular weight Heparin (LMWH) and intermittent pneumatic compression devices are strongly recommended to prevent thromboembolism. Dopamine agonists have been used to prevent and treat ovarian hyperstimulation syndrome by blocking expression of VEGF receptor [5]. There have been no significant pregnancy complications attributed to use of dopamine agonists, such as cabergoline, in early pregnancy [6]. Additionally, intensive care may be required for renal failure, pulmonary compromise, or life threatening thromboembolism [2].
Although few cases of spontaneous OHSS have been reported, multiple etiologies have been described. Both increased stimulation of normal ovarian FSH receptors and physiologic stimulation of mutated ovarian FSH receptors contribute to the pathologic recruitment of multiple dominant follicles. Increased circulating levels of FSH, caused by pituitary adenomas, have been identified as a primary cause of spontaneous OHSS [7-10]. These cases required treatment by transsphenoidal tumor resection for resolution of symptoms. Conversely, normal levels of circulating FSH may lead to OHSS through activation of mutated FSH receptors. These mutations were first identified in 2003, by both Vasseur et al. and Smits et al. [11,12]. Since then, new FSH mutations have been identified and additional cases of spontaneous OHSS described [13,14]. There are reports of both live births and spontaneous miscarriage in cases of FSH receptor mutations.
Increased FSH receptor stimulation may also result from binding domain similarities in FSH, thyroid stimulating hormone (TSH), and human chorionic gonadotropin (HCG). Elevated levels of TSH, found in hypothyroidism have been described in two prior cases of spontaneous OHSS [15,16]. The patients were started on levothyroxine and delivered at term. Similarly, elevated levels of HCG, as found in molar pregnancies or multiple gestations, have been found to stimulate normal ovarian FSH receptors and cause spontaneous OHSS [1,17-19]. The reported cases ended in spontaneous miscarriage or pregnancy termination. Finally, polycystic ovary syndrome (PCOS) predisposes patients to the development of multiple dominant follicles and has been attributed to spontaneous OHSS, even with physiologic levels of FSH, TSH, and HCG [20]. These physiologic mechanisms explain the timing of spontaneous OHSS, which occurs as physiologic levels of HCG rise, at 8-14 weeks. While multiple cases and etiologies have been described, there are few cases of recurrent, idiopathic spontaneous OHSS [3,4].
References
-
Rachad M, Chaara H, Zahra Fdili F, Bouguern H, Melhouf A (2011) Ovarian hyperstimulation syndrome in a spontaneous pregnancy with invasive mole: report of a case. Pan Afr med J 9: 23.
-
(2008) Ovarian hyperstimulation syndrome. Fertil Steril 90: 034.
-
Olatunbosun OA, Gilliland B, Brydon LA, Chizen DR, Pierson RA (1996) Spontaneous ovarian hyperstimulation syndrome in four consecutive pregnancies. Clin Exp Obstet Gynecol 23: 127-132.
-
Lovgren TR, Tomich PG, Smith CV, Berg TG, Maclin V (2009) Spontaneous severe ovarian hyperstimulation syndrome in successive pregnancies with successful outcomes. Obstet Gynecol 113: 493-495.
-
Soares SR (2012) Etiology of OHSS and use of dopamine agonists. Fertil Steril 97: 517-522.
-
Stalldecker G, Mallea-Gil MS, Guitelman M, Alfieri A, Ballarino MC, et al. (2010) Effects of cabergoline on pregnancy and embryo-fetal development: retrospective study on 103 pregnancies and a review of the literature. Pituitary 13: 345-350.
-
Baba T, Endo T, Kitajima Y, Kamiya H, Moriwaka O, et al. (2009) Spontaneous ovarian hyperstimulation syndrome and pituitary adenoma: incidental pregnancy triggers a catastrophic event. Fertil Steril 92: 390.e1-3.
-
Halupczok J, Kluba-Szyszka A, Bidzinska-Speichert B, Knychalski B (2015) Ovarian Hyperstimulation Caused by Gonadotroph Pituitary Adenoma--Review. Adv Clin Exp Med 24: 695-703.
-
Roberts JE, Spandorfer S, Fasouliotis SJ, Lin K, Rosenwaks Z (2005) Spontaneous ovarian hyperstimulation caused by a follicle-stimulating hormone-secreting pituitary adenoma. Fertil Steril 83: 208-210.
-
Murakami T, Higashitsuji H, Yoshinaga K, Terada Y, Ito K, et al. (2004) Management of ovarian hyperstimulation due to follicle-stimulating hormone-secreting gonadotroph adenoma. BJOG: an international journal of obstetrics and gynaecology 111: 1297-1300.
-
Vasseur C, Rodien P, Beau I, Desroches A, Gerard C, et al. (2003) A chorionic gonadotropin-sensitive mutation in the follicle-stimulating hormone receptor as a cause of familial gestational spontaneous ovarian hyperstimulation syndrome. N Engl J Med 349: 753-759.
-
Smits G, Olatunbosun O, Delbaere A, Pierson R, Vassart G, et al. (2003) Ovarian hyperstimulation syndrome due to a mutation in the follicle-stimulating hormone receptor. N Engl J Med 349: 760-766.
-
Dieterich M, Bolz M, Reimer T, Costagliola S, Gerber B (2010) Two different entities of spontaneous ovarian hyperstimulation in a woman with FSH receptor mutation. Reprod Biomed Online 20: 751-758.
-
Chauhan AR, Prasad M, Chamariya S, Achrekar S, Mahale SD, et al. (2015) Novel FSH receptor mutation in a case of spontaneous ovarian hyperstimulation syndrome with successful pregnancy outcome. J Hum Reprod Sci 8: 230-233.
-
Sridev S, Barathan S (2013) Case report on spontaneous ovarian hyperstimulation syndrome following natural conception associated with primary hypothyroidism. J Hum Reprod Sci 6: 158-161.
-
Rotmensch S, Scommegna A (1989) Spontaneous ovarian hyperstimulation syndrome associated with hypothyroidism. Am J Obstet Gynecol 160: 1220-1222.
-
Wu X, Zhu J, Zhao A (2015) Ovarian hyperstimulation syndrome in a spontaneous pregnancy with invasive mole. J Obstet Gynaecol Res 41: 817-822.
-
Davoudian P (2015) Placental mesenchymal dysplasia associated with spontaneous ovarian hyperstimulation syndrome. BMJ Case Reports.
-
Agrawal NR, Gupta G, Verma K, Varyani N (2012) Spontaneous ovarian hyperstimulation syndrome in a triplet pregnancy. Case reports in critical care 2012.
-
Zalel Y, Orvieto R, Ben-Rafael Z, Homburg R, Fisher O, et al. (1995) Recurrent spontaneous ovarian hyperstimulation syndrome associated with polycystic ovary syndrome. Gynecol Endocrinol 9: 313-315.





