Acute or delayed paraplegia or quadriplegia following anterior cervical discectomies and fusion are not common. We report this single case report of delayed quadriplegia following an anterior cervical discectomies and fusion without any change of sensory or motor evoked potentials during the operation.
The intraoperative somatosensory (SSEPs) were performed by stimulation of tibial nerves or the median (MN). Motor evoked potentials (TcMEPs) were recorded from intrinsic hand or foot muscles after delivering high voltage electrical pulses to the motor cortex.
We report a case of postoperative weakness which was followed by an incomplete quadriplegia in a patient after cervical discectomy and fusion. The intraoperative somatosensory (SSEPs) and TcMEPs recordings were normal throughout the surgery. Upon termination of the procedure and in the recovery room patient followed commands and was freely able to move all extremities. The weakness in the upper and lower limbs ensued within 20 minutes after which progressively turned into a severe weakness of upper limbs and complete motor paralysis in the lower limbs. The emergency MRI scan was not diagnostic at that point but the follow-up MRI scan a day after surgery demonstrated a multi-level spinal cord edema and infraction.
Spinal cord ischemia should be managed aggressively to improve spinal cord perfusion. The end prognosis depends on the severity of insult to neuronal tissue.
Postoperative neurologic deficits after cervical spine surgery are uncommon [1-6]. Causes of such deteriorations include surgical trauma, vascular compromise, inadequate cord decompression or spinal cord compression by epidural or subdural hematoma due to dislodgement of surgical construct [1,2,4,7,8]. Postoperative cervical cord infarction is a rare cause. Neuro-imaging is pivotal to exclude reversible causes and to confirm the presence of a spinal cord infarction [5,9,10].
Only a few cases of cervical spinal cord infarction after decompressive surgery have been reported [5,11]. The proposed causes of ischemic events included intraoperative or post-operative hypotension or decreased venous return.
After anterior or posterior decompression of the cervical spine [3]. Sometime, however, patients move their limbs freely right after surgery for a short time, then, for no obvious reason, paralysis suddenly develops. Such paraplegia often resolves within a few hours of timely diagnosis and treatment. At times, an immediate MRI scan fails to identify an explanation for this phenomenon. In contrast, the present case report identifies an immediate total paralysis of a patient who otherwise was moving four extremities upon termination of a multi-level cervical discectomy and fusion. The immediate MRI scan which was performed right after this event was not diagnostic. However, the follow-up MRI identified a massive multi-level spinal cord infarction a day after surgery.
This 40-year-old male had complained of leg and arm weakness and lack of coordination with some decreased sensation in both upper and lower extremities. MRI scan demonstrated extremely tight stenosis at C3-4, C4-5, and mildly severe stenosis at C5-6 level. With evidence of myelomalacia at C3-4 level. There was also thickening of the posterior longitudinal ligament (PLL) consistent with early ossification. The patient was admitted for surgical correction of the problem. Past medical history revealed a history of blurry revision, diabetes type 2, diabetic neuropathy, and cervical disc disease, myelopathy, hyperglycemia, and hypertension. The baseline blood pressure recording was 150/96 mmHg with mean of 114 mg before induction.
The patient underwent anterior cervical discectomy and fusion at C3-4, C4-5, and C5-6 levels with decompression of the spinal cord and C4-6 nerve roots followed by anterior interbody fusion with lordotic PEEK cages each level.
Intraoperative somatosensory (SSEPs) and motor evoked potentials (TcMEPs) were performed at the baseline and throughout the procedure all evoked potential findings were present but outside of the normal values (Figure 1).
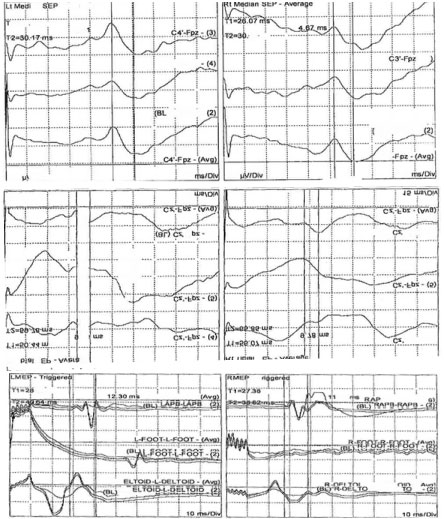 Figure 1: Cortical somatosensory (SSEPs) and transcranial motor evoked potentials (TcMEPs) recordings in this patient at the baseline (pre-incision). The SSEPs were recorded after the median and posterior tibial nerve stimulations on the right (right column) and left (left column) sides. The TcMEPs were recorded from the left (left column) and right (right column) abductor policies (AP), adductor halluces (AH) and deltoid (DL) muscles. View Figure 1
Figure 1: Cortical somatosensory (SSEPs) and transcranial motor evoked potentials (TcMEPs) recordings in this patient at the baseline (pre-incision). The SSEPs were recorded after the median and posterior tibial nerve stimulations on the right (right column) and left (left column) sides. The TcMEPs were recorded from the left (left column) and right (right column) abductor policies (AP), adductor halluces (AH) and deltoid (DL) muscles. View Figure 1
At the termination of the surgical procedure. The patient was extubated and upon recovery from anesthesia was able to move his extremities.
In the recovery room, patient was able to move upper and lower limbs for approximately 20 minutes. He then became progressively very weak in arms and essentially no movements in legs. Clinical examination revealed motor power of grade 0 bilaterally from L2 to S1. Post-anesthesia blood pressure recording ranged around 103/51 mmHg with lowest point at 89/42 mmHg at the recovery room.
Emergency MRI of the spine was non-diagnostic. It was decided the patient needed an immediate posterior decompression and fusion to further decompress the spinal cord. The patient underwent bilateral laminectomy at C3-4, C4-5, and C5-6 and placement of lateral mass screws and rods. Motor evoked potential (TcMEPs) monitoring at this point demonstrated loss of upper (abductor policies brevis = APB muscles) and lower (adductor halluces = AH muscles) extremity function. Only right side deltoid and biceps demonstrated some evoked EMG activity. The SSEPs were also absent from the upper and the lower limbs. A follow-up MRI next day showed abnormal signal intensity within the cord with postoperative changes at C3-4, C4-5, and C5-6 levels. The cervical spinal cord had developed extensive signal abnormality and cord expansion from C1 through C7. This was interpreted as cord edema and a multi-level cord infarct (Figure 2). There was no definite evidence of hemorrhage within the spinal cord. The patient was essentially remained paralyzed with severe pulmonary issues after this point.
 Figure 2: MRI spine cervical w/o contrast.
Figure 2: MRI spine cervical w/o contrast.
There are postoperative changes of ACDF at C3-4, C4-5, and C5-6 levels. The cervical spinal cord has developed extensive signal abnormality and cord expansion from C1 through C7. This could represent cord edema or an infarct. There was no definite evidence of haemorrhage within the spinal cord. View Figure 2
Hemodynamic infarction of the spinal cord has been a sporadic issue in the medical literature [12]. The present case is not the first one of its kind. In a report dealing with a case of spinal infarction having a similar pathology to the present one, a hypothesis was suggested stating that the order of vulnerability of the spinal cord is: 1 gray matter; 2 white matter adjacent to gray matter; and 3 peripheral zone of white matter [12]. Acute spinal cord infarction is uncommon accounting for 1.2% of stroke admissions. The vast majority of spinal infarction involves the anterior spinal artery and has a distinct clinical feature because of sparing posterior columns [12,13]. These patients have preserved posterior column function despite loss of pain and temperature with bilateral lower extremity weakness. Special attention should be paid to patients with lesions between C3 to C5 and T4 to T9. C3 to C5 supplies the phrenic nerve whereas T4 to T9 supplies the greater splanchnic nerve and therefore vasomotor tone [14]. If either level is involved, patient are at risks for respiratory failure or orthostatic hypotension.
Spinal cord ischemia is commonly seen after aortic procedures with a prevalence rate ranging 3-14% [15]. Other causes included atherosclerosis, cardiac embolism, epidural anesthesia and hypotension-induced surgery [15]. They highlight the importance of early diagnosis and suggest a management approach. Spinal cord ischemia is a diagnosis of exclusion. A high index of suspicion is required, especially since the MRI scan may be normal in the acute phase. Alblas, et al. reported that the focal cord swelling is typical of spinal cord ischemia manifest after 1-2 days of initial insult [16]. In our patient, the sudden deterioration of power in his limbs was unlikely to be caused by an intraoperative injury as the neuromonitoring signals remained constant throughout the surgery. The outcome of a transient spinal cord ischemia is usually favorable as is in some reported cases [7]. Jacob YL, et al. found similar results with a 71% return to ambulation in patients who had paraparesis. Spinal cord ischemia should be managed aggressively with medical treatment to improve spinal cord perfusion [14,15].
Our patient was diagnosed with spinal cord ischemia having excluded all other possible causes. Furthermore he was able to move his limbs with motor strength of grade 5 once he was extubated. The postoperative MRI image clearly showed a 3 level signal intensity change (stroke) within the spinal cord tissue. Hypotension seen in the recovery room was not the result of spinal cord injury "spinal shock" because patient upon recovery and extubation was fully alert and capable of moving 4 extremities. The post-operative emergency MRI helped to rule out the possibility of hematoma causing delayed spinal cord compression. His final diagnosis of spinal cord ischemia also correlated with his clinical manifestation of hypotensive shock. In conclusion, spinal cord ischemia post-surgery should be recognized early especially in the presence of hypotensive shock. Spinal cord ischemia should be managed aggressively with medical treatment to improve spinal cord perfusion. The prognosis depends on the severity of deficits and is usually favorable.