So far, there is no conventional parameters that have been proven to possess the ability to predict the histology of systemic lupus erythematosus (SLE). A few autoimmune serology markers of SLE including anti-dsDNA antibodies, complement C3 and C4, and anti-nucleosome could be helpful clinically, but the correlation between those and lupus renal disease is still imperfect. Recently, the urinary vascular cell adhesion molecule-1 (VCAM-1), kidney injury molecule-1 (KIM-1) and endothelin-1 (ET-1) have been studied for the diagnosis of lupus nephritis (LN). Nevertheless, it is still unknown whether the urinary VCAM-1, KIM-1 and ET-1 could be used as disease monitoring and flare predictor tools for LN.
To evaluate the levels of VCAM-1, KIM-1, and ET-1 in active LN, inactive LN and controls, cut-off points and diagnostic accuracy and their correlation with SLE Disease Activity Index (SLEDAI), renal SLEDAI (rSLEDAI), and the standard immunological markers.
This study involved 60 LN patients and 30 controls conducted in the Hospital Universiti Sains Malaysia (Hospital USM) from September 2016 to February 2018. All three biomarkers were determined by enzyme-linked immunosorbent assay (ELISA) in urine samples of patients. Receiver operating characteristic analysis was performed to obtain the best cut-off values and to calculate the performance of these markers. The correlation was done between urinary biomarkers and immunological parameters. Statistical analysis was performed using SPSS software, version 22.0.
Urinary VCAM-1, KIM-1, and ET-1 levels were significantly higher in active LN patients compared to inactive LN patients and controls. These markers correlated significantly with anti-dsDNA, complement C3, complement C4, urine protein/urine creatinine (Uprot/Ucreat), SLE disease activity index (SLEDAI), and renal SLEDAI scores. The urinary KIM-1 significantly correlated with all the immunological parameters except for complement C4. Urinary ET-1 showed higher specificity and sensitivity in differentiating LN patients and healthy controls (AUC 0.809) than urinary VCAM-1 (AUC 0.725) and urinary KIM-1 (AUC 0.640).
Our study demonstrated that urinary VCAM-1, KIM-1, and ET-1 might be potential biomarkers specific for the lupus renal disease.
Biomarker, Vascular cell adhesion molecule-1, Kidney injury molecule-1, Endothelin-1, Lupus nephritis
Systemic lupus erythematosus (SLE) is a heterogeneous autoimmune disease, of uncertain etiology, with numerous patterns of clinical manifestations due to the production of autoantibody. Some SLE patients have a high risk of developing lupus nephritis (LN) [1,2]. LN is one of a serious complication in SLE since it is the major forecaster of poor prognosis [3-5]. LN patients ultimately succumb to the end-stage kidney as well as cardiovascular damage [5,6].
Kidney biopsy, known to be the gold standard for the diagnosis of LN, is typically prompted by an abnormal urinary sediment, high proteinuria or serum creatinine [7]. However, it is an invasive procedure and impractical for monitoring treatment of LN. There remains a need for non-invasive determination of biomarkers associated with renal activity as well as predict renal flares. Nowadays, a myriad of potential novel biomarkers has been studied in LN [8-11]. Indeed, to be clinically practical, biomarkers of LN would need to be measured in the urine, since urine collection is less invasive compared to renal biopsies. Hence, novel biomarkers need to be explored specifically for early diagnosis of renal disease and the prediction of kidney flares in SLE.
VCAM-1 is an adhesion molecule originated from the immunoglobulin superfamily. It is crucial for the transmigration of immune cells from the circulation into tissues experiencing inflammatory processes [12]. VCAM-1 has been recognized to be increased in the sera, urine and kidneys of SLE patients and correlate well with the disease activity [13]. The production and serum levels of VCAM-1 are increased in patients with SLE and this is even more expressed in LN. Kidney injury molecule (KIM-1) is a type 1 membrane protein and expressed at very low levels in the normal kidney [14]. This molecule is associated with inflammation and renal interstitial fibrosis in certain types of renal diseases [15]. A previous study demonstrated that urinary KIM-1 levels were increased in chronic kidney disease (CKD) patients as well as those with LN and correlated with tubulointerstitial lesions [16]. Till now, it remains unknown whether KIM-1 could be potentially used as a parameter for the assessment of different kind of injuries in LN [14]. Meanwhile, Endothelin-1 (ET-1) is a 21-amino acid peptide, which is implicated in the development of CKD [17,18]. ET-1 is the most effective endogenous vasoconstrictor as well as being produced within the vasculature and the kidney [19,20]. Urinary ET-1 excretion is well-correlated with the production of renal ET-1 [21] and it self-regulate plasma ET-1 concentrations [17,19]. Prior study demonstrated elevated ET-1 in the plasma and urine in severe CKD patients [19,22]. However, there are no data available to emphasize the usefulness of urinary ET-1 as one of the potential biomarkers for LN.
Hence, the aim of the study is to evaluate the role of urinary VCAM-1, KIM-1, and ET-1 in predicting renal disease in LN as well as to analyse the correlation between the levels of these urinary markers with immunological parameters.
This was a cross-sectional study involving LN patients from the Hospital Universiti Sains Malaysia (Hospital USM). A total of 60 LN patients were enrolled in this study, 30 of which were diagnosed as having active LN, 30 with inactive LN and 30 controls. All LN patients were recruited from the Rheumatology Outpatient Clinic or the Medical Ward between January 2016 and December 2017. Relevant clinical data were gathered from the patients' medical records. All patients must satisfy the 21012 SLICC criteria [23] and aged between 18-56 years old. We exclude patients who are Hepatitis B or Hepatitis C positive; having chronic illnesses, e.g. diabetes mellitus and tuberculosis; having acute viral or bacterial infections e.g. flu or diarrhea; having autoimmune, allergic, or endocrine diseases; and prolonged treatment on corticosteroid or immunosuppressive drugs. The study protocols and written consent were approved by the ethics committee of Universiti Sains Malaysia (USM) according to the Declaration of Helsinki (USM/JEPeM/15060235).
In this study, SLE disease activity was assessed by the systemic lupus erythematosus disease activity index (SLEDAI), which is a validated tool to assess lupus activity. Patients with SLEDAI score ≥ 6 were defined as having active SLE disease and inactive SLE disease with SLEDAI score < 6 [24]. In addition, renal SLEDAI (rSLEDAI) was used to assess kidney disease activity for renal involvement. There are four kidney-related parameters, which are hematuria, pyuria, proteinuria, and urinary casts. Scores for the renal SLEDAI ranged from 0 (inactive renal disease) to a maximum of 16. Active LN are those with rSLEDAI scores of 4 or more [25].
Histological classes were characterized according to the International Society of Nephrology/Renal Pathology Society (ISN/RPS) 2003 reclassification. There are six histological classifications of LN: (i) Minimal mesangial LN, (ii) Mesangial LN, (iii) Focal LN, (iv) Diffuse proliferative LN, (v) Membranous LN, and (vi) Glomerulosclerosis [26].
Blood and urine samples were collected and allowed to clot prior to separation. Serum and urine obtained were aliquot and stored at -80 ℃ until test analysis. Anti-dsDNA antibody was semi-quantitatively determined using the Crithidia luciliae indirect immunofluorescence test (CLIFT) method (MBL, Japan). Anti-dsDNA with titers of 1:10 was considered positive and the results were reported in titers from 1:10 to 1:160. Serum C3 and C4 levels were considered low at levels less than 0.66 g/L and 0.20 g/L, respectively. The Uprot/Ucreat ratio helps to evaluate the excess protein in the urine, monitor the function of the kidney, and detects kidney damage. The urine samples for urinary VCAM-1, KIM-1 and ET-1 were measured using enzyme-linked immunosorbent assay (ELISA) kits from Elabscience Biotech, Wuhan, China. The values of the urinary markers were normalized to the urine creatinine.
Values were expressed as mean (standard deviation). The results were analysed by One-Way ANOVA (continuous variables) and chi-square (categorical variables) tests. One sample t test was used to compare the mean of one numerical data and independent t test to compare the means of a numerical variable between two independent groups. Correlation analysis between two variables was performed using the Pearson correlation coefficient and Spearman's rank correlation. Receiver operating characteristics (ROC) curve analysis was employed to study the best cut-off values of the protein markers and to see the ability of each marker in differentiating LN patients and normal controls.
Elevation of the urinary markers was defined based on the best cut-off values from ROC analyses. The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of each of the urinary markers were calculated using the 2 × 2 contingency tables. In all the analysis, p values lower than 0.05 were considered statistically significant. All data entry and statistical analysis were performed using the IBM© SPSS© Statistics version 22.
In total, 60 LN patients and 30 controls were enrolled in this study with 77 (85.56%) of the LN patients and controls were female while the remaining 13 (14.44%) were male. Majority of the LN patients were Malay (90.0%) and the remaining 10.0% from other ethnicities. The disease duration in active LN (80.97 [71.64]) and inactive LN (98.03 [91.11]) were significantly different with p < 0.001. There was a significant difference in the SLEDAI score. The SLEDAI score was significantly higher in the active LN groups compared to the inactive groups (active LN; 10.2 [5.5] versus inactive LN; 2.6 [2.5], p < 0.001). The score for rSLEDAI also exhibited significant results specifically in the active LN patients (6.93 [3.20], p < 0.001). Anti-dsDNA with the titer 1:10 was considered positive. Anti-dsDNA was positive in 18 (60.0%) of the active LN patients and 8 (26.7%) of the inactive LN patients. All controls were tested negative for anti-dsDNA. Meanwhile, C3 exhibited significantly low results in the active LN, 0.80 g/L (0.38 g/L); inactive LN, 0.99 g/L (0.25 g/L); and controls, 1.09 g/L (0.20 g/L); p = 0.001. Similarly, C4 also demonstrated low significant results in the active LN, 0.16 g/L (0.09 g/L); inactive LN, 0.23 g/L (0.11 g/L); and controls, 0.25 g/L (0.07 g/L); p = 0.001. The Uprot/Ucreat ratio was significantly higher in the active LN patients 2.32 (4.95) compared to the inactive LN patients 0.20 (0.32) and controls 0.05 (0.03) with p-value = 0.004. The demographic data for the patients are summarized in Table 1.
Table 1: Socio demographic, laboratory parameters, and disease activity of studied groups (n = 90). View Table 1
Renal biopsy was performed in all of the SLE patients. As shown in Figure 1, the most encountered type of renal disease was the diffuse proliferative glomerulonephritis (class II) (25 patients), followed by the mesangial proliferative LN (class I, 20 patients), the membranous LN (class IV, 12 patients), and the focal LN (class III, 3 patients).
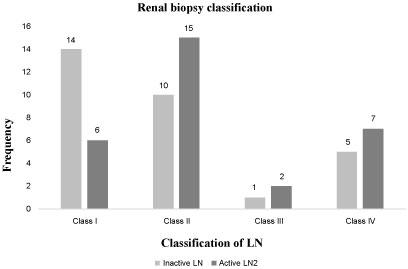 Figure 1: Renal biopsy classification of Lupus Nephritis patients. View Figure 1
Figure 1: Renal biopsy classification of Lupus Nephritis patients. View Figure 1
The mean (SD) of urinary VCAM-1 level was significantly higher in the active LN patients compared to the inactive group (6.54 [5.79] ng/mg Cr; 3.37 [1.44] ng/mg Cr; p = 0.005) and both were significantly higher than the level in the controls 2.93 (0.61) ng/mg Cr (p < 0.001). Meanwhile, the mean (SD) of urinary KIM-1 level was also significantly higher in the active LN patients as compared to the inactive LN group (0.27 [0.36] ng/mg Cr; 0.19 [0.17] ng/mg Cr; p = 0.040) and both were significantly higher than the level in the controls 0.11 [0.51] ng/mg Cr (p = 0.009). Consequently, the urinary ET-1 level in the active LN patients was significantly higher (4.29 [3.77]) pg/mg Cr compared to the inactive LN group with 2.86 (1.03) pg/mg Cr with p value 0.010 and the controls 0.89 (1.18) pg/mg Cr with a p value < 0.001. The means of urinary VCAM-1, KIM-1, and ET-1 between active LN, inactive LN, and controls are presented in Figure 2.
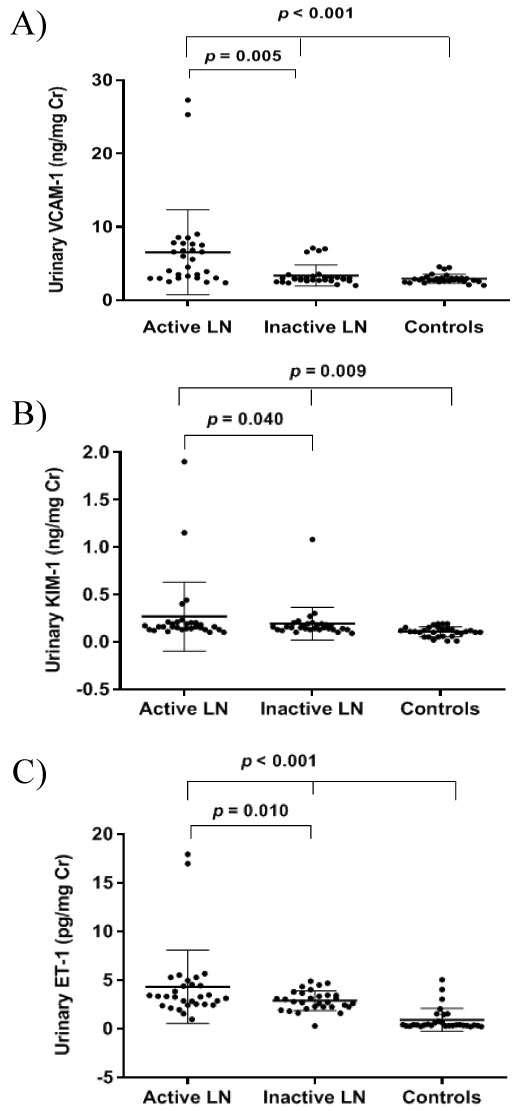 Figure 2: Mean of urinary VCAM-1 between active LN, inactive LN, and controls (A). Mean of urinary KIM-1 between active LN, inactive LN, and controls (B). Mean of urinary ET-1 between active LN, inactive LN, and controls (C) *p < 0.05 is significant. View Figure 2
Figure 2: Mean of urinary VCAM-1 between active LN, inactive LN, and controls (A). Mean of urinary KIM-1 between active LN, inactive LN, and controls (B). Mean of urinary ET-1 between active LN, inactive LN, and controls (C) *p < 0.05 is significant. View Figure 2
ROC curve analyses were accomplished to initiate the best cut-off values by measuring the sensitivity (true positive) and 1-specificity (false positive) of the protein markers. In this study, the ROC curve was performed to differentiate between LN patients and normal controls. The AUCs and the best cut-off values are shown in Table 2 and Figure 3. Among the three urinary markers, ET-1 demonstrated the highest AUC (0.809, 95% CI = 0.71-0.91, p < 0.001) and sensitivity or specificity in differentiating LN patients and normal controls followed by VCAM-1 (AUC 0.725, 95% CI = 0.62-0.83, p = 0.001) and KIM-1 (AUC 0.640, 95% CI = 0.49-0.78, p = 0.046). The cut-off values for urinary VCAM-1, KIM-1, and ET-1 were 3.6 ng/mg Cr, 0.14 ng/mg Cr and 1.9 pg/mg Cr. Positive (LN) is considered when the level is above the cut-off values and negative (not LN) is regarded when the level is below the cut-off values. Based on the cut-off values, urinary ET-1 showed the best sensitivity and specificity followed by urinary VCAM-1 and urinary KIM-1 for diagnosing LN.
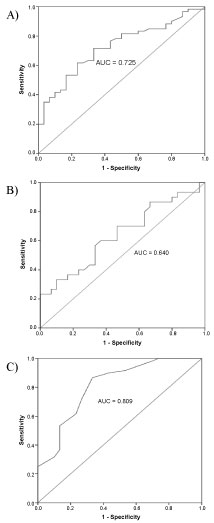 Figure 3: Area under the curve of urinary VCAM-1 (AUC 0.725, 95% CI = 0.62-0.83, p = 0.001) (A). Area under the curve of urinary KIM-1 (AUC 0.640, 95% CI = 0.49-0.78, p = 0.046) (B). Area under the curve of urinary ET-1 (AUC 0.809, 95% CI = 0.71-0.91, p < 0.001) (C). View Figure 3
Figure 3: Area under the curve of urinary VCAM-1 (AUC 0.725, 95% CI = 0.62-0.83, p = 0.001) (A). Area under the curve of urinary KIM-1 (AUC 0.640, 95% CI = 0.49-0.78, p = 0.046) (B). Area under the curve of urinary ET-1 (AUC 0.809, 95% CI = 0.71-0.91, p < 0.001) (C). View Figure 3
Table 2: Cut-off value and diagnostic accuracy of urinary VCAM-1, KIM-1, and ET-1 in diagnosing LN. View Table 2
All three urinary markers in patients with LN (n = 60) were correlated significantly with the anti-dsDNA (VCAM-1, Rho 0.401, p < 0.001; KIM-1, Rho 0.287, p = 0.006; ET-1, Rho 0.298, p < 0.001), Uprot/Ucreat ratio (VCAM-1, Rho 0.379, p < 0.001; KIM-1, Rho 0.342, p = 0.001; ET-1, Rho 0.500, p < 0.001), SLEDAI (VCAM-1, Rho 0.524, p < 0.001; KIM-1, Rho 0.480, p < 0.001; ET-1, Rho 0.504, p < 0.001) and rSLEDAI (VCAM-1, Rho 0.500, p < 0.001; KIM-1, Rho 0.332, p = 0.001; ET-1, Rho 0.440, p = 0.001). Meanwhile, these markers were inversely correlated with the serum C3 levels (VCAM-1, Rho -0.39, p < 0.001; KIM-1, Rho -0.232, p = 0.028; ET-1, Rho -0.203, p = 0.011) and serum C4 levels (VCAM-1, Rho -0.38, p < 0.001; ET-1, Rho -0.246, p < 0.019). However, the urinary KIM-1 was not significantly inversely correlated with serum C4 levels (Table 3).
Table 3: Correlation of urinary VCAM-1, KIM-1, and ET-1 with immunological parameters in LN patients. View Table 3
In this cross-sectional study, the mean age of active lupus nephritis (LN) patients was significantly lower than inactive LN patients. Most of the young SLE patients tend to be of newly diagnosed cases. Hence, more flares were seen in the younger age group of active LN patients. In this cohort, most of the LN patients were female (96.7%) which is consistent with other Malaysian cohorts of LN patients (96.9%) that demonstrated an approximately comparable proportion of female patients [27]. The reasons for this finding were due to the role of endogenous sex hormones in SLE-pathogenesis. Malaysia is a multiracial country which consists of Malay, Chinese, and Indian ethnicities [28]. In Malaysia, a prevalence of 43/100,000 individuals have been testified where the Chinese exhibited the highest prevalence of SLE followed by the Malays and Indians [29,30]. This study was conducted in the Kelantan state of Malaysia, where the population is predominantly Malay, hence the majority of the patients were Malays (54/60; 90.0%). In the present study, the mean difference of the SLEDAI score was statistically significant between the active LN patients and inactive LN patients. Similarly, the rSLEDAI score was significantly statistically different between both groups. Hence, this result justified the comparison of research interests between these two groups.
Current routine tests for determining SLE disease activity include anti-dsDNA antibody as well as serum C3 and C4 levels, which are also listed in the SLEDAI criteria. The prevalence of anti-dsDNA antibody in SLE patients ranged from 36% to 69% [31]. In this study, the anti-dsDNA antibody levels were positive in 18 (60.0%) LN patients. Active LN patients presented a higher frequency of anti-dsDNA as compared to inactive LN patients. Evidence from previous studies revealed that the presence of anti-dsDNA antibodies was a factor associated with the presence of nephritis, suggesting a predominant role of anti-dsDNA antibody in the disease profile regarding renal involvement. Complement C3 demonstrated significantly low results in active LN which is consistent with previous study [32]. Complement C3 is a good screening test for lupus patients with renal disease. Decreased levels of C4 in active LN is also consistent with the previous study by Hussain, et al. who reported that C4 levels were decreased during disease flare and provided an essential protective role against the development of LN. They stated that lower C3 levels significantly manifest a renal flare. A prior study reported that partial defects or homozygous defects in either C4A or C4B would result in the reduction of total C4 levels [5]. The Uprot/Ucreat ratio in the present study also demonstrated a similar trend with the previous study where high mean values were observed in the active LN group compared to the inactive LN and controls [7]. Hence, our finding suggests that the elevation of Uprot/Ucreat ratio exhibit association with the renal activity in patients with SLE and act as an indicator of renal damage.
Lupus nephritis (LN) was observed in 75%-80% of patients in previous studies [4]. There is a common agreement in the literature that the active classes of biopsy proven LN are class III, IV, V and VI while class I and II are considered less active, requiring limited immunosuppressive therapy [4,33]. In this study, class II was the most common class on the initial biopsy followed by class I, IV and III. Nevertheless, previous studies stated that class III and IV were the most common class on the initial biopsy [33-35]. The reason for the most common class II in our cohort LN might be due to an early diagnosis of LN and their class might change to a higher grade of nephritis. A prior study showed that most patients with class II exhibited transformation to the active class of nephritis (class III, IV, and V) [33].
In our cohort, the urinary levels of VCAM-1, KIM-1, and ET-1 were significantly higher in patients with active LN as compared to inactive LN and controls. The urinary levels of these markers correlated significantly with the anti-dsDNA, complement C3, complement C4, Uprot/Ucreat ratio, SLEDAI, and rSLEDAI, whereas, urinary KIM-1 correlated significantly with all of these immunological parameters except for complement C4. These markers were also able to differentiate between LN patients and normal controls.
VCAM-1 is a type of cell adhesion molecules. VCAM-1 levels were elevated in several autoimmune diseases including SLE as well as rheumatoid arthritis [36,37]. Previous studies have found that urinary VCAM-1 is a useful marker for SLE with renal involvement [7,9,38-40]. Our study demonstrated that urinary VCAM-1 level was significantly elevated in active LN as compared to inactive LN and controls. This finding is parallel to other studies that showed the elevated urinary VCAM-1 level in Hispanic, Caucasian and African Americans patients with LN than normal controls [7,13,41,42]. Moreover, urinary VCAM-1 levels also differed significantly between the active and inactive LN patients. These findings were in agreement with other studies that found a higher prevalence of urinary VCAM-1 in active LN than in inactive LN [7]. However, few studies demonstrated increased urinary soluble VCAM-1 level significantly correlates with the overall disease activity and damage, but not with active nephritis [37]. The results of the present study also confirmed the finding that urinary VCAM-1 is a potential marker that can differentiate LN patients from normal controls with a higher specificity or sensitivity than urinary KIM-1. In the present study, anti-dsDNA, complement C3, complement C4, Uprot/Ucreat ratio, SLEDAI and renal SLEDAI were also positively correlated with the urinary VCAM-1. Nevertheless, till now there is no single study focusing on the correlation of urinary VCAM-1 with anti-dsDNA. One of the earlier studies on adhesion molecules demonstrated similar findings with serum C3 and serum C4 [13]. These findings were consistent with the theory that immune complex formation and up-regulation of adhesion molecules are involved in LN pathogenesis. Besides, urinary VCAM-1 levels showed a fair correlation with concurrent Uprot/Ucreat ratio in our study. Notably, it has been reported that urinary VCAM-1 demonstrated a strong correlation with the disease activity as well as with the Uprot/Ucreat ratio [7,43]. Similarly, Kiani, et al. found that the urinary VCAM-1 had a strong correlation with descriptor of renal activity [38]. Thus, our study suggests that urinary VCAM-1 is associated with nephritis activity in SLE patients.
Kidney injury molecule-1 (KIM-1) is vastly expressed in the renal tubular cells after injury and is frequently regarded as an early biomarker for acute kidney injury (AKI) [44]. We found that urinary KIM-1 levels were significantly higher in active LN patients than those in inactive LN patients and controls with the lowest sensitivity and specificity of 56.7% and 65.0% among other urinary biomarkers in this study. Comparable with the previous study, urinary KIM-1 levels were significantly elevated in SLE patients with active LN as compared to inactive LN patients and normal controls [14]. Hence, the elevation of urinary KIM-1 in the present study might be the potential clinical measures specifically for the disease severity in LN patients. In the present study, urinary KIM-1 was able to differentiate LN patients from normal controls with an AUC of 0.640 and it was the lowest among all three biomarkers but still proven to be able to discriminate between LN patients and normal controls. It was observed that the urinary KIM-1 correlated significantly with anti-dsDNA, complement C3, Uprot/Ucreat ratio, SLEDAI and rSLEDAI but not with complement C4. This finding suggests that complement C4 was a less sensitive parameter of the disease activity. Parallel to our study, Mas, et al. showed that urinary KIM-1 correlated with the urinary Uprot/Ucreat ratio [45]. On the contrary, Nozaki, et al. reported that urinary KIM-1 was not correlated with the anti-dsDNA, complement C4, and total SLEDAI but significantly correlated with proteinuria and tubule damage in active LN group [14]. Meanwhile, no correlation among the urinary KIM-1 levels at baseline with the Uprot/Ucreat ratio after 6-8 months treatment [14]. However, there is still no study focusing on the correlation of the urinary KIM-1 with complement C3 and rSLEDAI, thus our finding could be considered as a novelty. Therefore, urinary KIM-1 might serve as one of the potential biomarkers for LN patients.
Endothelin-1 (ET-1) is encoded by the EDN1 gene and produced by vascular endothelial cells. ET-1 is intricate in the pathogenesis of CKD [19,46]. Our study showed a significant increase in the urinary ET-1 levels in active LN as compared to inactive LN and controls with the highest sensitivity and specificity of 80.0% and 96.7% respectively. The findings coincide with previous studies where urinary ET-1 levels were elevated in the LN patients and were expressed at low abundance in the normal human kidney [17,19,47]. Similarly, Dhaun, et al. concluded that ET-1 was implicated in the progression of kidney disease [19]. In our study, the urinary ET-1 showed significant correlation with the anti-dsDNA, complement C3, complement C4, Uprot/Ucreat ratio, SLEDAI, and rSLEDAI. Nevertheless, the prior study only highlighted the correlation of urinary ET-1 with SLEDAI. Notably, Tony, et al. stated that urinary ET-1 levels have significant correlation with SLEDAI [17]. To the best of our knowledge, none of the studies focuses on the correlation of the urinary ET-1 with the selected immunological markers as in the present study. Hence, the urinary ET-1 might be an appropriate non-invasive biomarker for monitoring disease activity as well as the possibility being a useful parameter for the renal inflammatory disease specifically in LN patients.
In this study, the limitation of LN patient's availability seen during a clinic day may influence the impact of our findings. Future studies might include a larger sample size with multicentre participation. For further investigations, we recommend that LN classes should be performed to determine whether these classes are particularly associated with the prognosis of kidney injury.
In conclusion, our study showed that urinary VCAM-1, and ET-1 were found to be significantly elevated in active LN patients and significantly correlated with the anti-dsDNA, complement C3, complement C4, Uprot/Ucreat ratio, SLEDAI as well as rSLEDAI. Meanwhile, the urinary KIM-1 was significantly correlated with all the selected immunological markers except for complement C4. The urinary ET-1 showed higher specificity and sensitivity in differentiating LN patients and controls compared to the urinary VCAM-1 and urinary KIM-1. Our results provide further evidence that urinary VCAM-1, KIM-1, and ET-1 could be potential biomarkers for lupus renal disease. However, further studies regarding a long-term renal disease, and prospective trials to see if elevated VCAM-1, KIM-1, and ET-1 levels could be a reliable predictor of disease flare, have not yet been accomplished.