Management of Immediate Complications Following Bariatric Surgery: Clinical Pearls for the Clinician Managing Bariatric Patients
Maher El Chaar1* and Keith Gersin2
1School of Medicine, Temple University, St Luke’s University Hospital and Health Network, USA
2Chief Bariatric Surgery, Carolinas Medical Center, University of North Carolina, USA
*Corresponding author:
Maher El Chaar, Clinical Associate Professor, School of Medicine, Temple University, St Luke’s University Hospital and Health Network, Co-Medical Director of Bariatric Surgery, Allentown, Pennsylvania, USA, E-mail: maher.elchaar@gmail.com
J Obes Weight-Loss Medic,
JOWM-1-003 (Vol 1, Issue 1),
Review Article
Received: March 01, 2015: Accepted: March 13, 2015: Published: March 16, 2015
Citation: Chaar ME, Gersin K (2015) Management of Immediate Complications Following
Bariatric Surgery: Clinical Pearls for the Clinician Managing Bariatric Patients. J Obes
Weight-Loss Medic 1:003
Copyright: © 2015 Chaar ME. This is an open-access article distributed under the terms
of the Creative Commons Attribution License, which permits unrestricted use, distribution,
and reproduction in any medium, provided the original author and source are credited.
Abstract
Bariatric surgery is the only effective long term treatment of morbid obesity. With the establishment of an accreditation process for bariatric centers and the development of laparoscopic approaches to bariatric surgery, in addition to fellowship training, bariatric surgery became a role model for other surgical specialties in terms of efficacy and safety. Bariatric surgery has now a long track record of safety and a very low morbidity and mortality rates. In addition, the number of bariatric procedures being performed is increasing dramatically. Health care providers caring for bariatric patients will encounter on occasions certain complications specific to bariatric patients in the immediate postoperative period. The objective of this review is to illustrate some of the immediate postoperative complications following bariatric surgery to provide general guidelines for a timely diagnosis and management of postoperative patients. These bariatric specific complications include gastrointestinal leakage, gastrointestinal bleeding and small bowel obstruction. We will also discuss the diagnosis of postoperative thromboembolic and cardiac diseases in bariatric patients.
Introduction
Bariatric surgery is the only effective and proven long-term solution for the treatment for morbid obesity [1]. The number of patients undergoing bariatric surgery has increased dramatically in the past two decades due in part to the adoption of laparoscopic techniques [2]. It is imperative that clinicians caring for post-operative bariatric patients have a clear understanding and high index of suspicion for the potential complications that may arise following weight loss surgery. Failure to quickly and correctly diagnose these complications may lead to devastating outcomes, including death.
Post-operative bariatric complications may be arbitrarily grouped into those occurring immediately following surgery (within the first 24-72 hours following surgery) and those occurring later (months to years). Immediate complications related to the surgical technique itself and occurring immediate after surgery include gastrointestinal leakage, bleeding, small bowel obstructions (intra-luminal or extra-luminal in origin) venous thromboembolic disease and cardiac events. Complications occurring later include hernias (internal and incisional), symptomatic cholelithiasis, gastroesophageal reflux disease, strictures, stenosis, weight regain and nutritional deficiencies. Although venous thromboembolic diseases and cardiac complications are not unique to weight loss surgery, they may occur with greater frequency in the morbidly obese and are therefore included here [3]. In this review article we will focus on the immediate complications occurring after bariatric surgery and provide general guidelines as to the work-up and management of those complications.
Gastrointestinal Leakage
Gastrointestinal (GI) leakage is one of the most devastating complications that can be encountered following bariatric surgery. The possible sites of leakage are primarily from staple lines however leaks can result from any anastomosis whether stapled, hand sewn or a combination of the two (Figure 1). In addition, missed serosal injuries or enterotomies at the time of surgery are another likely source. GI leakage usually occurs in the immediate postoperative period but rarely may present several weeks following surgery and usually results in increased morbidity and mortality. The incidence of GI leakage following gastric bypass surgery ranges between 1% and 5% with rates following revisional bariatric surgery even higher. [5-7]. The incidence of GI leakage following sleeve gastrectomy ranges from 1.2% to 2.7% usually resulting from failure of the gastric sleeve staple line [8]. The differences in leakage rates between open and laparoscopic techniques remain controversial. Some authors have suggested that the leakage rates after laparoscopic roux-en-y-gastric bypass (LRYGB) are higher than when performed in an open fashion possibly due to surgeon experience and learning curve [9]. DeMaria et al. demonstrated a reduction in leakage rates from 6.8% to 1.8% after performing their first 102 LRYGB cases [10]. When performed by experienced surgeons, Nguyen et al. found no difference in the rate of postoperative anastomotic leakage rates between open and laparoscopic groups [11].
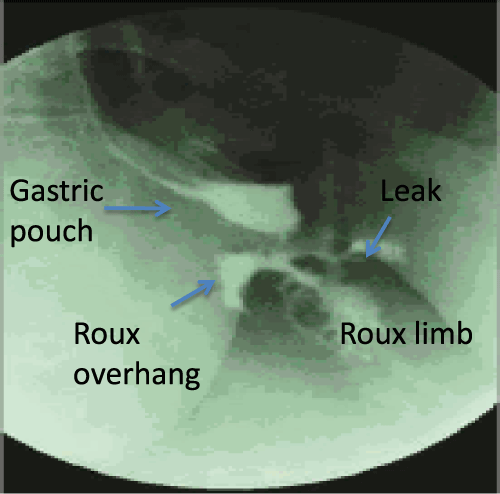
Figure 1: UGI demonstrating a leak from the gastrojejunostomy anastomosis
1 day following a Laparoscopic Roux-en-Y-Gastric Bypass. Patient signs and
symptoms include fever, tachycardia and abodominal pain.
View Figure 1
The use of routine post-operative contrast studies to rule out GI leakage remains controversial. Selective imaging based upon the patient’s clinical situation is acceptable [12]. Unstable patients with a suspicion of GI leakage should be explored immediately without the need of any additional testing or radiographic studies. Two commonly performed diagnostic procedures include upper gastrointestinal series (UGI) and computerized axial tomography (CT) scanning. The sensitivity of these modalities varies depending upon patient related factors and the experience of the interpreting radiologist. Limitations of UGI series involve the inability to visualize all potential sources of GI leaks and therefore CT scanning is more sensitive in this regard. A recent retrospective analysis of prospectively collected data on 3,018 patients, demonstrated that leaks were detected in 17 of 56 patients (30%) when UGI series were used and in 28 of 50 patients (56%) when CT scans were used [6]. Another advantage of abdominal and pelvic CT scanning is the ability to reveal other intra-abdominal pathology that share similar clinical symptoms to GI leakage such as small bowel obstruction or intra-abdominal bleeding. A potential limitation of CT scans is the inability to perform the procedure due to weight limitations or inability of patients to fit inside the scanners.
Clinicians should not rely on radiographic studies alone in determining which patients require operative interventions. A negative UGI or CT scan does not rule out the presence of GI leakage ad in the appropriate clinical setting in the presence of signs and symptoms suggestive of GI leakage, a diagnostic laparoscopy or laparotomy should be performed [9]. Patients with GI leakage may present with one or more of the following signs and symptoms: fever, tachycardia, tachypnea, hypoxia, oliguria, abdominal, back or shoulder pain, feeling of impending doom and hypotension.
Basic surgical tenants should be followed to appropriately manage these patients requiring exploration. Exploration can be performed laparoscopically or in an open fashion. Laparoscopic exploration may be technically challenging even for experienced laparoscopic surgeons because of bowel distension and acute inflammation leading to adhesions and ablation of tissue planes. At the time of exploration, all staple lines in addition the gastrojejunostomy (GJ) and jejunojejunostomy (JJ) anastomosis should be carefully inspected to help localize the site of leak. Gentle, blunt dissection can minimize serosal injuries or enterotomies to the gastric pouch or the roux limb secondary to the severe inflammatory reaction. An intra-operative endoscopy can be very useful in localizing the site of leak. When a leak is identified, suture repair may be attempted and/or omental patching if possible to cover the site of leakage. Abdominal washout and wide drainage is also recommended [13]. If the site of leakage is not identified, abdominal washout and wide drainage is recommended. At the conclusion of exploration, surgeons should consider the placement of a gastrostomy tube for decompression and feeding when warranted.
Staple line disruption and leakage after performance of a sleeve gastrectomy can develop in the immediate postoperative period or several days later. In the immediate postoperative period, patients with leakage following sleeve gastrectomy should be explored, drained and the site of leakage identified and suture repaired [14]. In the later postoperative period patients may be managed by endoscopic stent placement combined with laparoscopic or percutaneous drainage of abdominal collections [15,16]. Gastric outlet obstruction secondary to a leak and subsequent stenosis may require a revision to a roux-en-y gastric bypass [17].
In stable patients with a controlled leak, non-operative management may be considered. These patients may have had a drain placed at the time of initial operation or subsequently via interventional radiologic techniques. Patients who are managed non-operatively are treated with antibiotic administration and parenteral nutrition and should be explored immediately if they fail to improve clinically, develop new symptoms or any signs of sepsis [18]. Endoscopic means to manage leaks following bariatric surgery are preferentially performed in specialized large volume, tertiary care centers under approved clinical trials and are not currently considered standards of care.
Gastrointestinal Bleeding
The rate of gastrointestinal bleeding (GIB) following bariatric surgery varies in the literature between 1 and 4% [55]. GIB usually arises from staple lines, however may also be the result of injury to intra-abdominal organs including the liver and spleen. A potential mechanism of staple line bleeding is the penetration of the mucosa by the stapler or the transaction of a vessel traversing the serosal layer of the stomach or small bowel [19].
Familiarity with the presentation of GIB following bariatric surgery is a necessity for those caring for these patients. GIB following bariatric surgery can be either intra-luminal, intra-abdominal (Figure 2) or both. In patients with signs and symptoms of increased abdominal pressure, shoulder pain, tachycardia, and decreased urine output, intra-abdominal GIB should be considered in addition to consideration of a gastric leak. Furthermore, patients with intra-luminal bleeding may present with hematemesis when the bleeding originates from the gastrojejunostomy anastomosis. Signs of bowel obstruction may also be present if the intra-luminal bleeding and subsequent clot formation obstructs the bowel at the jejuno-jejunostomy. The use of laboratory studies may help confirm the presence of a GIB, however a normal hemoglobin and hematocrit obtained shortly after surgery may not be reliable or reflective of acute post-surgical bleeding. A simple history can also help localize the site of bleeding in bariatric patients without the need of invasive diagnostic tests or imaging studies. For example, the site of bleeding in a patient presenting with hematemesis is more likely the staple line of the gastric pouch of the gastrojejunostomy anastomosis whereas a patient presenting with blood per rectum is most likely bleeding from the newly formed jejenujejunostomy or the gastric remnant staple line [19].
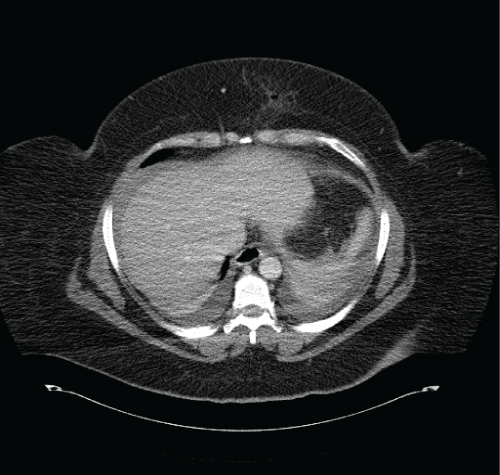
Figure 2: CT scan demonstrating hemoperitoneum 1 day following a
Laparoscopic Roux-en-Y-Gastric Bypass (LRYGB). The patient developed
abdominal pressure, tachycardia and a drop in hemoglobin following LRYGB
View Figure 2
The management of post-operative GIB in bariatric patients may be different than that of general surgery patients [20]. Bariatric patients presenting with post-operative GIB, are best managed in the operating room. Intubation is recommended to secure the airway, help prevent aspiration and quickly convert to operative interventions if required. The use of bedside, therapeutic endoscopy is to be discouraged in this patient population.
Management of unstable patients with GIB includes the placement of large bore intravenous lines and fluid and blood resuscitation. Intra-operative endoscopy is a useful adjunct, both diagnostically and therapeutically. In addition in helping localize bleeding, many endoscopic modalities (clip application, epinephrine injection, electrocautery) can successfully control GIB and obviate the need for surgical intervention. Intra-luminal clots should be evacuated to allow endoscopic visualization and treatment and to help prevent the development of gastrointestinal obstruction from future clot migration. Surgical exploration, (either open or laparoscopic), may be indicated when the source of bleeding cannot be identified. At the time of exploration, intra-luminal or intra-abdominal clots should be evacuated and the staple lines over sewn [19,21,22]. The additional need for placement of gastrostomy tubes for decompression or feeding remains controversial.
The management of GIB in stable patients may be resuscitative only. In a recent report, 450 consecutive patients who underwent LRYGB over a 30-month period at the Cleveland Clinic Florida were retrospectively reviewed. Twenty patients (4.4%) developed an acute postoperative bleed and among those only three patients (15%) were unstable and required an operation. The hemodynamically stable patients were managed successfully with non-procedural measures including 15 patients (75%) who required blood transfusions [23]. Stable patients who subsequently manifest ongoing bleeding or become unstable should be intubated and treated endoscopically, surgically or a combination of both.
Small Bowel Obstruction
Small bowel obstruction (SBO) following bariatric surgery can lead to staple line breakdown and leakage, bowel necrosis and even death. The incidence of small bowel obstruction ranges from 1.5% to 11% following LRYGB [24]. SBO can result from incisional hernias, internal hernias, adhesions, cicatrisation of the roux-limb at the level of the mesocolon, intra-luminal blood clots, intussusception of the roux-limb and technical issues associated with the construction of the jejunojejunostomy (JJ) [24,25] .
SBO following bariatric surgery can be divided into early and late obstruction. While some authors have attempted to standardize the definition of “early” and “late” obstructions [25], there is no clear consensus. Bariatric patients presenting with SBO in the immediate post-operative period usually report abdominal discomfort, bloating, nausea and vomiting. This usually results, in the immediate post-operative period, from technical issues with the construction of the JJ, trocar site hernias or bleeding with intra-luminal blood clots. Internal hernias can also result in SBO but they usually present later and those will be discussed in other chapters [26,27]. Bilious vomiting usually indicates obstruction distally in the common channel (Figure 3) while non-bilious vomiting is more indicative of a proximal obstruction (Figure 4). These patients may be tachycardic and hypoxic and therefore gastrointestinal leakage and bleeding must also be considered.
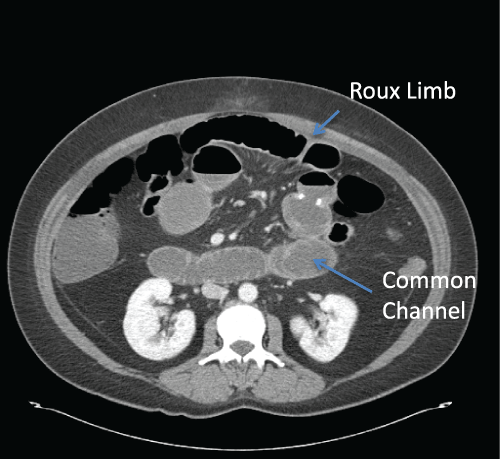
Figure 3: CT scan demonstrating dilated common channel and Roux limb
following a Laparoscopic Roux-en-Y-Gastric Bypass. This patient presented
with abdominal pain and bilious vomiting.
View Figure 3
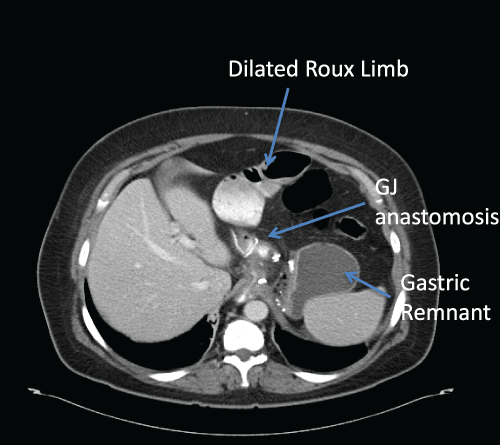
Figure 4: CT scan demonstrating dilated Roux limb secondary to an
obstruction of the JJ. This patient developed abdominal pain, nausea and
non-bilious vomiting two days following the Laparoscopic Roux-en-Y-Gastric
Bypass.
View Figure 4
Diagnosis of SBO should be confirmed in a timely fashion to prevent bowel necrosis or staple line breakdown and leakage. Physical examination may be unreliable as abdominal distension is difficult to assess in the morbidly obese patients [28,29]. CT scanning is usually performed when SBO is suspected and can frequently demonstrate the area of obstruction. Even with a negative CT scan, surgeons should have a low threshold for performing a diagnostic laparoscopy on those patients when there is a high index of suspicion of SBO to prevent bowel necrosis.
The management of the different causes of immediate post-operative SBO will be discussed separately.
First, SBO resulting from herniation thru trocar sites is managed like in any general surgery patients. The herniated bowel should be reduced and the defect closed. The presence of bowel necrosis following SBO necessitates bowel resection and reanastomosis. In the past, it was suggested that closure of any trocar larger than 5mm is necessary to prevent herniation. Today, with the advent of the new dilating trocars, there is no need for fascial closure of any trocar site that is 12 mm or less. However, many surgeons continue to close any trocar site that is larger than 5 mm. It’s important to note that even though it’s extremely difficult to make the diagnosis of trocar site herniation by physical exam because of the bariatric patient body habitus, the diagnosis is easily made using CT scanning [30-32].
Second, SBO resulting from gastrointestinal hemorrhage and the presence of intraluminal blood clot should follow the same guidelines for managing bariatric patients with GI hemorrhage. As previously mentioned, the bleeding site should be controlled and the intra-luminal clot evacuated thru an enterotomy or gastrotomy depending on the site of bleeding to relieve the SBO [19,22].
Third, obstruction at the JJ can result from narrowing of the anastomosis, edema, hematoma or acute angulation (Figure 3). In addition, bleeding at the JJ can also result in an intraluminal blood clot and a secondary SBO as previously mentioned. Obstruction at the JJ usually presents early but can also be seen within 7 to 19 days following the index operation [29]. Adherence to sound surgical principles and achieving hemostasis at the time of the creation of JJ can prevent bleeding, edema or the formation of hematoma. Acute angulation at the JJ can be prevented by the placement of one or two anti-obstruction stitches (Brolin Stitch) to approximate both limbs of the JJ anastomosis [33]. To prevent stenosis at the JJ, which usually results from closing the common enterotomy with a linear stapling device, some authors have suggested suture closure of the enterotomy. Another suggestion is the use of a new technique called “bidirectional firing” or “triple-stapling technique”. Using this technique, a surgeon fires two separate cartridges in opposite directions to create the anastomosis and then the common enterotomy is closed with a third linear firing [34-36]. The management of SBO secondary to obstruction at the JJ usually requires reexploration and revision of the JJ anastomosis.
Another uncommon reason for SBO following LRYGB is the Roux-en-O configuration in which the inappropriate identification of the roux limb and the bilio-pancreatic limb can result in the connection of the bilio-pancreatic limb to the gastric pouch. If the improper connection is not identified intraoperatively and addressed, it may result in symptoms and signs of small bowel obstruction in the postoperative period. Those patients usually present in the late postoperative period [37].
Venous Thrombo-Embolic Disease
Venous thrombo-embolic disease (VTE) is a complication that may present in the early post-operative period following bariatric surgery. The incidence has been reported to be 1% with no statistical differences observed between open RYGB, LRYGB and laparoscopic adjustable gastric banding (LAGB) procedures [38]. The diagnosis and management of VTE following bariatric surgery is similar to any surgical procedure and not unique to weight loss surgery. It is worth noting however, that the differential diagnosis of a patient presenting with hypoxia, shortness of breath, tachycardia and impending sense of doom following bariatric surgery should include, in addition to pulmonary embolus (PE), gastrointestinal leakage and bleeding. CT angiography (CTA) has become the diagnostic test of choice for the evaluation of patients with suspected PE unless the patient has a contraindication to receiving intravenous contrast or exceeds the weight limit of the scanner [39,40].
The focus of VTE as relates to bariatric surgery patients, lies in its’ prevention. To date, there is no consensus or standard of care when it comes to VTE prophylaxis. Some clinicians prefer chemoprophylaxis, pneumatic compression devices or a combination of the two to aid in the prevention of VTE [41]. The American Society for Metabolic and Bariatric Surgery (ASMBS) clinical issues committee published a position statement in June 2007, regarding prophylactic measures to reduce the risk of VTE in bariatric surgery patients. The position statement was later revised in 2013. In its guidelines, obese patients undergoing bariatric surgery were noted to be at moderate to high risk for VTE and therefore preventive measures in the peri-operative period were recommended. The use of lower extremity sequential compression devices and early ambulation were recommended and deemed appropriate for all bariatric patients when clinically feasible. In addition, routine administration of chemoprophylaxis, when not contraindicated, was recommended in addition to mechanical prophylaxis, for all bariatric surgery patients but the choice, dosage and duration of anticoagulation administration remain controversial. Also, in low risk patients the use of mechanical prophylaxis alone was shown to result in low risk of VTE (< 0.45).Individual practices were also encouraged to develop and adhere to protocols for the prevention of VTE [42].
The use of inferior vena cava (IVC) filters pre-operatively to prevent post-operative PE also remains controversial. In 2008 and 2009, the American Association of Clinical Endocrinologists (AACE), the Obesity Society (TOS) and the ASMBS published a set of recommendations regarding their use. These societies recommend that bariatric patients at risk for, or with a history of, deep venous thrombosis (DVT) or cor pulmonale should undergo an appropriate diagnostic evaluation for DVT. In addition, investigators recommended a prophylactic vena caval filter for patients with a history of prior PE, immobility, BMI>55, prior ileo-femoral DVT, evidence of venous stasis, known hypercoagulable state, pulmonary hypertension, obesity hypoventilation increased right-sided heart pressures [43,44]. Other studies, however, have shown a high incidence of device related complications [4]. Additionally data from the Bariatric Outcomes Longitudinal Database (BOLD) showed that IVC filter resulted in higher incidence of VTE [45]. The latest AMBS position statement on IVC filter placement recommend against the routine use of IVC filters. However, IVC filter placement can be considered in addition to mechanical and chemical prophylaxis in selected high risk patients [42].
Cardiovascular Disease
Obesity is a well-known independent risk factor for cardiovascular events. One reason bariatric patients with cardiac risk factors seek bariatric surgery is to decrease their overall long-term cardiovascular risk. Patients undergoing bariatric demonstrated a reduction in cardiovascular events and deaths [46-49]. To determine the safety and efficacy of bariatric surgery in obese patients with documented coronary artery disease (CAD), the rates of in-hospital cardiovascular complications and mortality of 52 patients with clinical CAD were compared with those of 507 patients without CAD. The rate of cardiovascular complications was 5.8% for patients with documented CAD and 1.4% for patients without CAD (p=0.06). The authors concluded that bariatric surgery could be safely performed in patients with documented CAD [50]. The Longitudinal Assessment of Bariatric Surgery (LABS) consortium performed a prospective, multicenter, observational study of 30-day outcomes in consecutive patients undergoing first-time bariatric procedures at 10 clinical sites in the United States between 2005 and 2007. The 30-day mortality rate among the 4776 patients included in the study was 0.3% [38]. Morino M et al. studied 13,871 patients who underwent bariatric surgery between January 1996 and January 2006 and reported sixty-day mortality rate of 0.25%, 17.6% of which were cardiac the deaths [51].
Given the low mortality of patients with CAD following bariatric surgery and the clear benefit of bariatric surgery in reducing the overall rate of cardiovascular events and deaths, it’s generally recommended to offer bariatric surgery for patients with CAD after a cardiac evaluation [50].
According to the American College of Cardiology/American Heart Association ACC/AHA 2009 guidelines, bariatric surgery is an intermediate risk surgery and all patients with active cardiac disease should undergo noninvasive testing. For patients with no active cardiac disease, and given the fact that bariatric surgery is an intermediate risk surgery, the functional status of the patient should be assessed. Patients with good functional status and functional capacity greater or equal to 4 (Metabolic Equivalents) METs without symptoms should proceed to surgery. However, it’s usually difficult to assess functional capacity in bariatric patients because of their weight and limited activity and therefore risk stratification should be performed to determine the presence or absence of clinical risk factors, which include diabetes mellitus, renal insufficiency, compensated or prior heart failure and ischemic heart disease. If the patient has no clinical risk factors then he or she can proceed to surgery. In the presence of 1 or more risk factors it’s recommended by the AHA to proceed with non-invasive testing or surgery with heart rate control with beta- blockers [52].
Conclusion
Clinicians caring for bariatric surgical patients need to have a clear understanding as to the potential complications that may arise in the immediate postoperative period. Although weight loss surgery is safe with a low mortality, delay in the recognition of complications may lead to increased morbidity and mortality rates. Many of the complications mentioned in this chapter present with similar signs and symptoms; therefore a heightened awareness as to the differential diagnosis is imperative. CT scanning is a useful adjunct in the diagnosis of many of these complications. Finally, prevention of these complications is equally as important as prompt recognition and treatment.
References
-
Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, et al. (2009) The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess 13: 1-190, 215-357, iii-iv.
-
Nguyen NT, Root J, Zainabadi K, Sabio A, Chalifoux S, et al. (2005) Accelerated growth of bariatric surgery with the introduction of minimally invasive surgery. Arch Surg 140: 1198-1202.
-
Poirier P, Alpert MA, Fleisher LA, Thompson PD, Sugerman HJ. et al. (2009) Cardiovascular Evaluation and Management of Severely Obese Patients Undergoing Surgery: A Science Advisory From the American Heart Association. Circulation 120: 86-95.
-
Birkmeyer NJ, Share D, Baser O, Carlin AM, Finks JF, et al. (2010) Preoperative placement of inferior vena cava filters and outcomes after gastric bypass surgery. Ann Surg 252: 313-318.
-
Podnos YD, Jimenez JC, Wilson SE, Stevens CM, Nguyen NT (2003) Complications after laparoscopic gastric bypass: a review of 3464 cases. Arch Surg 138: 957-961.
-
Gonzalez R, Sarr MG, Smith CD, Baghai M, Kendrick M, et al. (2007) Diagnosis and contemporary management of anastomotic leaks after gastric bypass for obesity. J Am Coll Surg 204: 47-55.
-
Fernandez AZ Jr, DeMaria EJ, Tichansky DS, Kellum JM, Wolfe LG, et al. (2004) Experience with over 3,000 open and laparoscopic bariatric procedures: multivariate analysis of factors related to leak and resultant mortality. Surg Endosc 18: 193-197.
-
Brethauer SA, Hammel JP, Schauer PR (2009) Systematic review of sleeve gastrectomy as staging and primary bariatric procedure. Surg Obes Relat Dis 5: 469-475.
-
Lee S, Carmody B, Wolfe L, Demaria E, Kellum JM, et al. (2007) Effect of location and speed of diagnosis on anastomotic leak outcomes in 3828 gastric bypass cases. J Gastrointest Surg 11: 708-713.
-
DeMaria EJ, Sugerman HJ, Kellum JM, Meador JG, Wolfe LG (2002) Results of 281 consecutive total laparoscopic Roux-en-Y gastric bypasses to treat morbid obesity. Ann Surg 235: 640-645.
-
Nguyen NT, Goldman C, Rosenquist CJ, Arango A, Cole CJ, et al. (2001) Laparoscopic versus open gastric bypass: a randomized study of outcomes, quality of life, and costs. Ann Surg 234: 279-289.
-
Lee SD, Khouzam MN, Kellum JM, DeMaria EJ, Meador JG, et al. (2007) Selective, versus routine, upper gastrointestinal series leads to equal morbidity and reduced hospital stay in laparoscopic gastric bypass patients. Surg Obes Relat Dis 3: 413-416.
-
Yurcisin BM, DeMaria EJ (2009) Management of leak in the bariatric gastric bypass patient: reoperate, drain and feed distally. J Gastrointest Surg 13: 1564-1566.
-
Csendes A, Braghetto I, León P, Burgos AM (2010) Management of leaks after laparoscopic sleeve gastrectomy in patients with obesity. J Gastrointest Surg 14: 1343-1348.
-
Nguyen NT, Nguyen XM, Dholakia C (2010) The use of endoscopic stent in management of leaks after sleeve gastrectomy. Obes Surg 20: 1289-1292.
-
Tan JT, Kariyawasam S, Wijeratne T, Chandraratna HS (2010) Diagnosis and management of gastric leaks after laparoscopic sleeve gastrectomy for morbid obesity. Obes Surg 20: 403-409.
-
Bellorin O, Lieb J, Szomstein S, Rosenthal RJ (2010) Laparoscopic conversion of sleeve gastrectomy to Roux-en-Y gastric bypass for acute gastric outlet obstruction after laparoscopic sleeve gastrectomy for morbid obesity. Surg Obes Relat Dis 6: 566-568.
-
Csendes A, Burdiles P, Burgos AM, Maluenda F, Diaz JC (2005) Conservative management of anastomotic leaks after 557 open gastric bypasses. Obes Surg 15: 1252-1256.
-
Nguyen NT, Longoria M, Chalifoux S, Wilson SE (2004) Gastrointestinal hemorrhage after laparoscopic gastric bypass. Obes Surg 14: 1308-1312.
-
Monkhouse SJ, Morgan JD, Norton SA (2009) Complications of bariatric surgery: presentation and emergency management--a review. Ann R Coll Surg Engl 91: 280-286.
-
Steffen R (2003) Early gastrointestinal hemorrhage after laparoscopic gastric bypass. Obes Surg 13: 466.
-
Nguyen NT, Rivers R, Wolfe BM (2003) Early gastrointestinal hemorrhage after laparoscopic gastric bypass. Obes Surg 13: 62-65.
-
Mehran A, Szomstein S, Zundel N, Rosenthal R (2003) Management of acute bleeding after laparoscopic Roux-en-Y gastric bypass. Obes Surg 13: 842-847.
-
Hwang RF, Swartz DE, Felix EL (2004) Causes of small bowel obstruction after laparoscopic gastric bypass. Surg Endosc 18: 1631-1635.
-
Nguyen NT, Huerta S, Gelfand D, Stevens CM, Jim J (2004) Bowel obstruction after laparoscopic Roux-en-Y gastric bypass. Obes Surg 14: 190-196.
-
Higa KD, Ho T, Boone KB (2003) Internal hernias after laparoscopic Roux-en-Y gastric bypass: incidence, treatment and prevention. Obes Surg 13: 350-354.
-
Garza E Jr, Kuhn J, Arnold D, Nicholson W, Reddy S, et al. (2004) Internal hernias after laparoscopic Roux-en-Y gastric bypass. Am J Surg 188: 796-800.
-
Gunabushanam G, Shankar S, Czerniach DR, Kelly JJ, Perugini RA (2009) Small-bowel obstruction after laparoscopic Roux-en-Y gastric bypass surgery. J Comput Assist Tomogr 33: 369-375.
-
Koppman JS, Li C, Gandsas A (2008) Small bowel obstruction after laparoscopic Roux-en-Y gastric bypass: a review of 9,527 patients. J Am Coll Surg 206: 571-584.
-
Felsher J, Brodsky J, Brody F (2003) Small bowel obstruction after laparoscopic Roux-en-Y gastric bypass. Surgery 134: 501-505.
-
Bhoyrul S, Payne J, Steffes B, Swanstrom L, Way LW (2000) A randomized prospective study of radially expanding trocars in laparoscopic surgery. J Gastrointest Surg 4: 392-397.
-
Chiong E, Hegarty PK, Davis JW, Kamat AM, Pisters LL, et al. (2010) Port-site hernias occurring after the use of bladeless radially expanding trocars. Urology 75: 574-580.
-
Brolin RE (1995) The antiobstruction stitch in stapled Roux-en-Y enteroenterostomy. Am J Surg 169: 355-357.
-
Frantzides CT, Zeni TM, Madan AK, Zografakis JG, Moore RE, et al. (2006) Laparoscopic Roux-en-Y Gastric bypass utilizing the triple stapling technique. JSLS 10: 176-179.
-
Madan AK, Frantzides CT (2003) Triple-stapling technique for jejunojejunostomy in laparoscopic gastric bypass. Arch Surg 138: 1029-1032.
-
Ahmad A, Cho K, Brathwaite C (2004) A technique of enteroenterostomy to prevent alimentary limb obstruction in laparoscopic Roux-en-Y gastric bypass. J Am Coll Surg 198: 159-162.
-
Sherman V, Dan AG, Lord JM, Chand B, Schauer PR (2009) Complications of gastric bypass: avoiding the Roux-en-O configuration. Obes Surg 19: 1190-1194.
-
Longitudinal Assessment of Bariatric Surgery (LABS) Consortium, Flum DR, Belle SH, King WC, Wahed AS, et al. (2009) Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med 361: 445-454.
-
Nikolaou K, Thieme S, Sommer W, Johnson T, Reiser MF (2010) Diagnosing pulmonary embolism: new computed tomography applications. J Thorac Imaging 25: 151-160.
-
Hartmann IJ, Wittenberg R, Schaefer-Prokop C (2010) Imaging of acute pulmonary embolism using multi-detector CT angiography: an update on imaging technique and interpretation. Eur J Radiol 74: 40-49.
-
Clements RH, Yellumahanthi K, Ballem N, Wesley M, Bland KI (2009) Pharmacologic prophylaxis against venous thromboembolic complications is not mandatory for all laparoscopic Roux-en-Y gastric bypass procedures. J Am Coll Surg 208: 917-921.
-
American Society for Metabolic and Bariatric Surgery Clinical Issues Committee (2013) ASMBS updated position statement on prophylactic measures to reduce the risk of venous thromboembolism in bariatric surgery patients. Surg Obes Relat Dis 9: 493-497.
-
Mechanick JI, Kushner RF, Sugerman HJ, Gonzalez-Campoy JM, Collazo-Clavell ML, et al. (2008) American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery Medical Guidelines for Clinical Practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Surg Obes Relat Dis 4: S109-184.
-
Mechanick JI, Kushner RF, Sugerman HJ, Gonzalez-Campoy JM, Collazo-Clavell ML, et al. (2009) American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Obesity (Silver Spring) 17 Suppl 1:S1-70.
-
Li W1, Gorecki P, Semaan E, Briggs W, Tortolani AJ, et al. (2012) Concurrent prophylactic placement of inferior vena cava filter in gastric bypass and adjustable banding operations in the Bariatric Outcomes Longitudinal Database. J Vasc Surg 55: 1690-1695.
-
Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, et al. (2004) Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 351: 2683-2693.
-
Batsis JA, Romero-Corral A, Collazo-Clavell ML, Sarr MG, Somers VK, et al. (2007) Effect of weight loss on predicted cardiovascular risk: change in cardiac risk after bariatric surgery. Obesity (Silver Spring) 15: 772-784.
-
Batsis JA, Sarr MG, Collazo-Clavell ML, Thomas RJ, Romero-Corral A, et al. (2008) Cardiovascular risk after bariatric surgery for obesity. Am J Cardiol 102: 930-937.
-
Jazet IM, de Groot GH, Tuijnebreyer WE, Fogteloo AJ, Vandenbroucke JP, et al. (2007) Cardiovascular risk factors after bariatric surgery: Do patients gain more than expected from their substantial weight loss? Eur J Intern Med 18: 39-43.
-
Lopez-Jimenez F, Bhatia S, Collazo-Clavell ML, Sarr MG, Somers VK (2005) Safety and efficacy of bariatric surgery in patients with coronary artery disease. Mayo Clin Proc 80: 1157-1162.
-
Morino M, Toppino M, Forestieri P, Angrisani L, Allaix ME, et al. (2007) Mortality after bariatric surgery: analysis of 13,871 morbidly obese patients from a national registry. Ann Surg 246: 1002-1007.
-
Fleischmann KE, Beckman JA, Buller CE, Calkins H, et al. (2009) 2009 ACCF/AHA focused update on perioperative beta blockade. J Am Coll Cardiol 54: 2102-2128.





