Obesity is recognized as a disease entity due to the damage that dysfunctional abdominal visceral fat can cause. Body shape and fat distribution are more predictive of future disease than Weight and Body Mass Index (BMI). An apple-shaped figure, as opposed to a pear-shaped figure, correlates to a greater Waist-To-Hip ratio and greater health risk for Type 2 Diabetes Mellitus (T2DM). The Centers for Disease Control defines obesity in relation to the BMI. However, the BMI can overestimate obesity since it neglects accounting for lean body muscle. The Waist-To-Hip Ratio (WHR) and Waist Circumference (WC) are better alternatives than the BMI to measure body shape. An innovative taxonomy called the 'Riopelle Fat Type Level' has been proposed to describe the hybrid measurements of WHR and WC, relate them to disease risk, and provide a motivational strategy to help middle-aged men navigate towards healthier lifestyles and longevity.
Body mass index, Body shape, Diabesity, Prediabetes, Type 2 diabetes, Waist circumference, Waist-To-Hip ratio
Body shape and fat distribution are more predictive of future disease than are Weight and Body Mass Index (BMI) [1-5]. An apple-shaped figure, as opposed to a pear-shaped figure, correlates to a body silhouette with a greater Waist-To-Hip ratio and greater health risk for metabolic syndrome, T2DM, heart disease, dyslipidemia, sleep apnea, nonalcoholic liver disease, cancer, and other comorbidities [1,4]. In the U.S. two-thirds of the population are either overweight or obese; of these individuals, 80-90% are already diabetic or prediabetic [6,7]. Diabesity is a new term recognized within the last few decades to describe how intertwined and metabolically damaging these two diseases are [8,9]. Since 1980, the rates of diabesity in the US have more than doubled; men of normal weight and without diabetes are now in the minority [1]. There is a lack of knowledge in the literature about what evidence-based approaches will help motivate overweight males to start and continue on a weight loss regimen [10]. Though the literature indicates that there are effective weight loss methods, there is often a lack of adequate attention and preparation from the primary care setting [11]. Frequently, educational strategies can be too complex or esoteric for patients to grasp their true intent, inhibiting positive behavior changes. In turn, this can result in unmotivated or confused at-risk patients unable to make much needed behavior changes [12].
Abdominal visceral adiposity is currently recognized as a primary contributing factor to the rising incidence of metabolic disease [13-15]. Accurate, yet convenient and economical methods to measure central fat are under scrutiny due to the multitude of currently available tools to measure obesity and body composition [16]. While methods such as the magnetic resonance imaging computerized tomography scans, dual energy x-ray absorptiometry and total body water scans report visceral abdominal fat with greater reliability, there is a lack of easy to use and cost-effective obesity evaluation measures for use on a wide scale basis for the busy primary care clinician. Anthropometric measurements of body composition, specifically central, truncal adiposity are commonly used in clinical settings and offer a good proxy for estimating obesity and its related comorbidities [16]. While there is potential for intra-rater and inter-rater error with anthropometric measurements, they offer an inexpensive and easy to learn methodology that can be implemented across broad ranges of clinical settings and research settings enforcing its practical applications for assessment [16].
The purpose of this review is to introduce and examine the utility of implementing an innovative, simple tool that is based on anthropometric measures of both the WHR and the WC called the 'Riopelle Fat Type Level', to help educate and motivate overweight males who are prediabetic or diabetic. The Riopelle Fat Type Level is derived from a person's WHR and WC and consists of six levels, e.g., 1-6 (Appendix A and Appendix B). The greater the WHR and WC, the greater the Riopelle Fat Type Level and subsequent risk for disease. Many studies report that the greater the WHR and WC, and hence the Riopelle Fat Type Level, the greater the risk for metabolic disease, including T2DM and cardiovascular disease [3,16,17]. Use of the BMI as a screening tool for obesity has been the "gold standard" in recent decades, frequently used in both research and clinical settings. The BMI offers a quick and easy assessment for generalized fatness, but can frequently under- or over-estimate the degree of obesity and associated risk for comorbidities. All measurements including the BMI, WHR, WC, and the newly proposed 'Riopelle Fat Type Level' have advantages and disadvantages, and vary in their precision of estimating adiposity [18].
Appendix A: Identification of weight status and associated health risk by fat-type level, waist-to-hip ratio, and body shape in men. View Appendix A
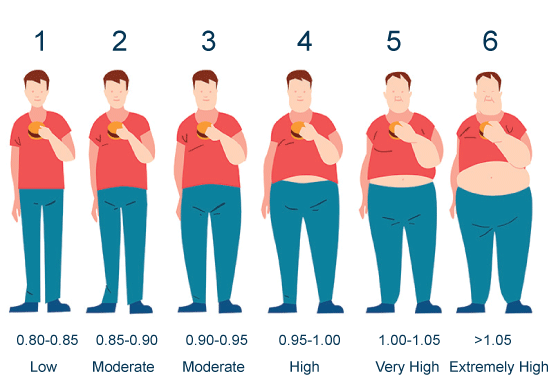 Appendix B: Fat type level: Waist-hip ratio & associated risk. View Appendix B
Appendix B: Fat type level: Waist-hip ratio & associated risk. View Appendix B
The BMI has been refuted in the literature as having flaws in both under-reporting and over-estimating disease risk, especially for men and older individuals [19-21]. Since the BMI is based solely on height and weight of the individual, it can lead to errors in defining obesity as it doesn't distinguish lean muscle from adipose tissue [21]. The WHR and WC offer a more focused estimate of abdominal obesity than the BMI. While the WHR and WC measure both intra-abdominal and total abdominal fat above the muscle, they are only a rough estimate of the metabolically damaging visceral fat [14]. Studies have been mixed on the validity of using the WHR and WC, but these measurements still provide better proxies to help estimate obesity-related disease risk than the BMI [14,16,22,23] In addition, the visual picture of excessive abdominal fatness can provide a superior motivating tool to help counsel men to lose weight (Appendix B).
Aging men are at greater risk for obesity than are aging women: 74% versus 64%, respectively [24]. Older men are more likely to develop excessive truncal and visceral fat, thus increasing diabetes risk [25]. Specifically, overweight men with a waist size larger than 102 centimeters (cm), have a 10-year incidence of T2DM and are 22 times more likely to develop diabetes than are men with normal BMIs (18.5 kg/m2to 22.4 kg/m2) and smaller waist sizes (< 93 cm) [18]. High concentration of visceral fat exposes the liver to higher degrees of free fatty acids, which impair the liver's ability to metabolize fatty acids and other compounds [26]. This leads to increased levels of apolipoprotein B and insulin, which lead in turn to hypertriglyceridemia and hyperinsulinemia [13]. Men with elevated fat percentages above 23.5% but of normal weight were seven times more likely to have metabolic syndrome [20,21]. Dysfunctional central fat is associated with increased risk for T2DM, fatty liver and cardiovascular disease [19]. A decrease in physical activity leads to a decrease in basal metabolic rate, a 40-50% decrease in lean muscle mass, and a consequential decrease in calorie-burning ability [27]. Further in the older population, the BMI is often inaccurate as it can over-report disease risk due to the loss of stature in aging [19]. Thus, risk factors increase at a greater rate in aging men in comparison to both women of comparable age and younger men [24].
Primary Care Providers (PCPs) often use various methods, such as weight; BMI; circumferences of waist, hip, neck, chest or thigh; and ratios such as Waist-To-Hip (WHR), Waist-To-Height, and Waist-To-Chest to help diagnosis and educate men about weight and health risk [3,11,16,17]. Common anthropometric measures include weight; BMI; various circumferences such as waist, hip, neck, chest and thigh various ratios including Waist-To-Hip, Waist-to-Height, Waist-to-Chest, and skin-fold thicknesses [28]. Other more cumbersome and costly, yet discriminating, measures include magnetic resonance imaging computerized tomography scans, dual energy x-ray absorptiometry and total body water or hydrometry which estimates body fluid distribution, fat mass, fat free mass, and bone [1,3]. However, these tools can often be confusing for patients and clinicians to understand due to many technical classifications and a lack of a universally and well-accepted diagnostic measure to evaluate weight and health risk [16,29]. Since most comorbid diseases resulting from being overweight and obese are asymptomatic in early stages, it is even more imperative that PCPs accurately assess and intervene early in men's lives to prevent future health problems and help fight the US obesity epidemic [11].
Diabetes is a chronic and progressive disease characterized by the body's inability to produce or use insulin effectively [6]. Dysregulation of insulin and/or glucose uptake results in hyperglycemia, which in turn causes tissue damage and complications of the disease. Unfortunately, many at-risk patients remain undiagnosed for ten to twenty years during which long-term injury is already occurring [6]. Diagnosis of prediabetes and T2DM is measured by laboratory and clinical assessment (Appendix C) [6]. The ADA recommends that adults over 40-years-old have a screening blood test to detect hyperglycemia every two to three years after the age of 40 or, depending on other risk factors, at a younger age. Outcomes for monitoring glycemic control include weight, BMI, WC, and WHR, triglycerides, fasting plasma glucose, and glycated hemoglobin [6]. Complications of T2DM can be reduced by intervening before its onset [30]. Even modest changes in weight and exercise can result in improvements in glycemic control [31,32].
Appendix C: Criteria for the diagnosis of prediabetes and diabetes. View Appendix C
A major opportunity exists to control the rising incidence of T2DM impacting patients' quality of life and healthcare systems [30]. Ali, et al. [33] reported that 33-49% of patients in the U.S. do not meet targets for glycemic, blood pressure, or cholesterol control. According to Dunkley, et al. [30] and Paulweber, et al. [34], there is substantial evidence to show that the rates and complications of diabetes are reduced by intervening before its onset. Several seminal, high-grade scientific trials demonstrate that modest changes in diet, weight, and physical activity result in improvements of more than 50% in parameters of glycemic control among individuals at risk [35-37]. In the Diabetes Prevention Program (DPP), researchers compared using lifestyle interventions to metformin [36,38]. They found that, in the lifestyle intervention group, patients were able to reduce their risk of developing T2DM by 58%, while the metformin group reduced risk by only 31% (p < 0.001) [36]. In the landmark Finnish Diabetes Prevention Study, Tuomilehto, et al. [32] reported that lifestyle intervention reduced the incidence of diabetes by 58% (p < 0.001), compared to the control group.
Early intervention also has a lasting effect on diabetes risk reduction. In a study by Li, et al. [39], there was a 43% decrease in the conversion to diabetes at 20 years. In a study by the Finnish Diabetes Prevention Trial, there was a 43% reduction in conversion at 7 years [37]; in a study by the U.S. Diabetes Prevention Program, conversion to diabetes was reduced 34% at 10 years [38]. These results provide good evidence that primary care clinicians can have a great effect on diabetes prevention.
The Centers for Disease Control [40] defines obesity in relation to the BMI, which has been the accepted "gold standard" for measuring weight, first introduced over two hundred years ago by Quetelet [3]. BMI is defined as mass in kilograms divided by height in meters squared [28]. According to data from the Prospective Studies Collaboration, which tested over 900,000 participants, there is a 30% increase in all-cause mortality for every increase of 5 units above a BMI of 25 kg/m2[41]. However, there are limitations to calculating obesity using BMI. An individual with excess fat but normal weight may be overlooked as overweight or obese; conversely, a person with a high BMI but low body fat may be diagnosed as obese [18]. Using BMI has been an easy, economical way to measure adiposity in a variety of settings. However, there are limitations to using BMI, particularly in epidemiological research; it can overestimate obesity because it does not take into account lean muscle or bone mass [18,42]. Men who have more lean muscle may be classified as being overweight or obese when, in actuality, they are otherwise healthy adults [3].
Since it is expensive and impractical to do magnetic resonance imaging, computed axial tomography, or bone densitometry on most patients, there is a need to find a reliable proxy to complement the deficiencies of the BMI [16]. While the WC and the WHR are not exact measures of visceral abdominal fat, they are reliable proxies for screening patients who present to the PCPs office with weight and obesity-related concerns. The WHR and the WC are trustworthy substitutes for BMI and can provide men with a realistic and practical picture of their health status [4,16,43,44]. Taken together, the WHR and WC are better alternatives than the BMI for measuring body shape, adiposity, determine health risk [4,45]. Both the WHR and WC are simple and inexpensive methods that correlate well with more precise imaging studies of dysfunctional truncal fat [45]. The greater the WHR, the greater the risk of metabolically damaging fat located in the abdominal area [46]. The use of a simple and innovative tool, called "Riopelle Fat Type Level", to help educate and motivate overweight males with prediabetes or diabetes has the potential to provide an accurate, cost-effective assessment of obesity.
Fat Type Levels, a novel taxonomy, was developed by the first author and has been used in a primary care clinic since 2013 in over 800 patients [47] (Appendix A). The taxonomy is based on identifying body shape, that is, apple versus pear shape, as defined by Savard [4]. The classification converts WHR and WC to a score from 1 to 6 and assigns a disease risk to the score. The taxonomy was developed as a way of categorizing overweight and obesity metrics into a simpler and more comprehensible format to promote more effective patient education and motivation by the healthcare practitioner. Terms used to describe weight, such as BMI, WC, or WHR and their scales, may be confusing for patients to understand and retain. A simple value of 1 through 6 is easier to understand. The 1 to 6 scale is based on WHR and WC values, which have been shown to correlate well with disease risk and body composition [4,48,49].
Data from the Prospective Studies Collaboration [48] reported a strong linear relationship between BMI and mortality, but there is an even stronger correlation between truncal fat and mortality [50,51]. The greater the WC, the greater the likelihood of visceral fat versus subcutaneous fat, which is less metabolically dysfunctional, Subcutaneous fat tends to be predominate in individuals who carry more weight around their hips and thighs than around their abdomens, reflecting a lower waist-to-hip ratio or a pear-shaped figure [2,19,48].
With advancing knowledge about adiposity, there is a need to further define how fat tissue functions as an endocrine organ influencing glucose and lipid metabolism and promotes damaging inflammation in the body [19]. Fat tissue is an endocrine organ and responds to different stimuli. Abdominal adipocytes hypertrophy with weight gain, while subcutaneous femoral fat cells become hyperplasic in response to weight gain [25]. There is a strong correlation between hypertrophied fat cells, inflammation, dyslipidemia, and insulin resistance [20]. The term adiposopathy or 'sick fat' refers to the pathogenic actions fat can have in the body. Adiposopathy is a direct result of fat distribution, composition, and percentage, rather than just weight in pounds or kilograms and BMI parameters [16,19]. It offers a much more sophisticated view of defining those in need of lifestyle or medical intervention [16]. Recognizing the problem is the first step for both the patient and the healthcare provider [52]. Primary prevention needs to start at the earliest stage to avert progression of this potentially deadly disease and can occur through diet, exercise, weight loss medications, bariatric surgery, and behavioral changes [16].
Obesity and diabetes prevention and intervention can occur at key opportunities, such as in the primary care office during a routine office visit or an annual physical [53]. The PCP has the unique advantage of seeing patients for various health concerns, creating many opportunities to address their weight issues [11]. Evidence shows that if health providers diagnose, recommend, and counsel clients about weight loss, patients will be more likely to follow a weight loss program [10,54,55]. Despite its well-established benefits, only 20-40% of primary care providers offer weight loss counseling to obese patients because of time constraints, inadequate knowledge, ill-prepared counseling skills, and difficulty explaining technical language [52].
Changes in body fat distribution with weight gain or weight loss have become targets for debate in recent years. Therapeutic goals for weight reduction often include WC and WHR reductions, as well as lowering BMI. There has been strong epidemiological evidence linking inflammatory illnesses such as T2DM and cardiovascular disease to obesity, but there is still debate on the best measure to assess adiposity and risk. Robust evidence and practicality of using anthropometric measures of WHR, WC, and hence 'Riopelle Fat Type Levels', suggests that abdominal adiposity may provide a more reliable and motivating marker for assessing and counseling patients about obesity than the BMI. A more comprehensive, yet practical, evaluation of fat type and metabolic activity needs to be recognized to enable more effective and efficient interventions at the level of both populations and individuals. The 'Riopelle Fat Type Level' incorporating the WHR and the waist circumference was created to close this gap and offer a simpler, economical tool to use in PCP offices to help motivate at-risk pre-diabetic or diabetic middle-aged persons to lose weight.