Studies in Cameroon reported high prevalence of urinary abnormalities in sickle cell anemia (SCA). There is a lack of data in this setting on the prevalence of chronic kidney disease (CKD) in SCA and Sickle cell trait (SCT).
Assess the prevalence and associate factors of CKD in people with sickle cell disease.
This was a cross-sectional study of six months durations (April-September 2017) involving SCA (HB SS) and SCT (HBAS) subjects at Douala Laquintinie hospital. CKD was diagnosed and classified according to 2012 Kidney Disease Improving Global Outcomes (KDIGO) criteria. Only patients with persistent urinary anomalies or decreased eGFR at 3 months were considered to have CKD.
We included 107 subjects among which 81 SCA (62% males) and 26 SCT (46% males) with a mean age of 19.5 ± 10 and 35.8 ± 7.8 years (p < 0.001) respectively. Compare to SCT, SCA subjects had lower body mass index and systolic blood pressure. Overall, CKD was found in 16 patients (15%): 13 (16%) patients in the SCA group and 3 patients (11%) in the SCT group. CKD frequency was comparable in both groups (p = 0.76). CKD was found in 37% of adult SCA patients. Albuminuria was more common in the SCA group [12 (15%) vs. 1 (4%) patient, p = 0.018]. Age ≤ 25 years was protective factor for both CKD (OR = 0.20 [0.003-0.135], p < 0.001) and albuminuria (OR = 0.23 [0.004-0.124], p < 0.001) in SCD group.
CKD in sickle cell disease is highly prevalent young adult in our setting.
Sickle cell anemia, Sickle cell trait, Chronic kidney disease, Age
BMI: Body Mass Index; CI: Confidence Interval; CKD: Chronic Kidney Disease; DBP: Diastolic Blood Pressure; eGFR: Estimated Glomerular Filtration Rate; Hb: Hemoglobin; HBV: Hepatitis Virus; HCV: Hepatitis Virus; HIV: Human Immunodeficiency Virus; LHD: Laquintinie Hospital of Douala; NSAID: Non-Steroidal Anti-Inflammatory Drug; SBP: Systolic Blood Pressure; SCD: Sickle Cell Disease; SCA: Sickle Cell Anemia; SCT: Sickle Cell Trait; SD: Standard Deviation; VOC: Vaso-Occlusive Crisis
Sickle cell disease (SCD) is common and life-threatening haematological disorder that affects millions of people worldwide [1,2]. It is caused by a mutation in the gene encoding the β-globin chain of hemoglobin, leading to the formation of hemoglobin S. SCD may occur as a homozygous inheritance of hemoglobin S (HbS) or and heterozygous inheritance of HbS with other β-globin mutations (e.g. HbSC) or even quantitative mutations that result in decreased or absent β-globin synthesis (e.g. thalassemia). Equatorial Africa is the bedrock of SCD in the world, probably because of survival advantage to patient with sickle cell trait (SCT), the heterozygote form HbAS, in region of endemic malaria [1]. Sickle cell anemia (SCA), the homozygote sickle cell disease, is still a life-threatening and debilitating condition, with high mortality rates recorded even in high income countries such as USA, where the median survival of SCA patients is 40-50 years [3]. This low survival is mainly due to multiple organ complication due to long standing microvascular obstruction. Chronic kidney disease (CKD) is a well-known and one of the most serious complication of SCA. Renal abnormalities start in childhood. The primum movens being microvascular obstruction causing an ischemic glomerular and/or tubular injury resulting in cortical infarction, papillary necrosis, focal segmental glomerulosclerosis, tubular atrophy and interstitial fibrosis [4]. The main clinical syndromes associated with these include proteinuria, hematuria, glomerular hyperfiltration, impaired urine concentration ability, renal tubular acidosis, CKD and end stage kidney disease [4-6]. Although SCT has been usually considered as a benign condition, renal complications are commonly reported and included impaired urinary concentration, asymptomatic hematuria, and papillary necrosis [7-9]. Moreover, recent studies underline SCT as a risk factor of chronic kidney disease in Africans Americans [10]. In a Cameroon, Kaze, et al. reported high frequency of proteinuria (94%) compare to other African studies [11,12]; other kidney abnormalities were leucocyturia (78%), hematuria (14%) and low estimated glomerular filtration rate (1.5%). However, the exact prevalence of chronic kidney disease in SCA is not known since the persistence of these anomalies at 3 months was not evaluated. Moreover, the prevalence of renal dysfunction in people with SCT in Cameroon has not been studied. This study was design to determinate the prevalence of CKD in people with SCA and SCT in the main SCD health facility of the economic capital of Cameroon.
We conducted a cross-sectional study from April 1st to September 30th 2017 in the' Emmanuel Billong center of Laquintinie hospital of Douala (LHD). LHD is one of biggest hospital of the country and it is located in Douala, the economic capital of Cameroon. The Emmanuel Billong unit is the main dedicated center for patient with sickle cell disease of the littoral region; receiving more than 100 patients per month; adults as children. It had a capacity of 18 beds and it held by one pediatric assisted by a general practitioner, a psychologist and 8 nurses. Participants were recruited during outpatient consultation. All SCA patient aged of at least 10 years consulted in the center during the study period were included. SCT patients in the family (parents or dibbling) of SCA patients of more than 10 years and who give their contentment were also recruited. Parental consent was obtained for all pediatric patients before inclusion. Patient with vaso-occlusive crisis (VOC), fever, macroscopic hematuria or signs of urinary tract infection were all excluded.
CKD was diagnosed and classified according to 2012 KDIGO criteria [13]. Glomerular filtration rate (GFR) was estimated using creatinine based equation: Bedside Schwartz equation for pediatric patient and chronic kidney disease epidemiology collaboration (CKD-EPI) equation for patient older than 18 years [12]. Patients who present urinary anomalies (proteinuria, leucocyturia or hematuria) or decrease eGFR (eGFR < 60 ml/min/1.73 m2) was reevaluated after 3 months. Only patients with persistent urinary anomalies or decreased eGFR at 3 months were considered to have CKD. CKD was classified as follow:
o Stage 1: eGFR ≥ 90 ml/min/1.73 m2 and urinary abnormalities
o Stage 2: 60 ≤ eGFR < 89 ml/min/1.73 m2 and urinary abnormalities
o Stage 3a: 45 ≤ eGFR < 60 ml/min/1.73 m2 with or without urinary abnormalities
o Stage 3b: 30 ≤ eGFR < 45 ml/min/1.73 m2 with or without urinary abnormalities
o Stage 4: 15 ≤ eGFR < 30 ml/min/1.73 m2 with or without urinary abnormalities
o Stage 5: eGFR < 15 ml/min/1.73 m2 with or without urinary abnormalities
eGFR = 41.3 × [height (m)/serum creatinine (mg/dl)]
eGFR = 141 × min (Scr/κ, 1)α × max (Scr/κ, 1-1.209 × 0.993Age × 1.018 [if female] × 1.159 [if black]
Scr = Serum creatinine (mg/dL),
κ is 0.7 for females and 0.9 for males,
α is -0.329 for females and -0.411 for males,
min indicates the minimum of Scr/κ or 1,
max indicates the maximum of Scr/κ or 1.
Baseline characteristics of patient were recorded in the medical field and information was complete during an interview with the patient and/or the parent. The following data were collected:
• Demographic and socio-economic data: age, sex, occupation and educational level.
• SCD data: Episode of VOC during the last year, baseline hemoglobin level, number of transfusion per year, prior hospitalizations, chronic complications (retinopathy, osteonecrosis, leg ulcer), usual medications (non-steroidal anti-inflammatory drug –NSAID, folic acid, hydroxyurea, herbal plant, and other).
• Clinical data: Weight, height, blood pressure (BP). The BP was measured after at least 5 minutes of rest with an automated oscillometric blood pressure measuring devices. The patient was asked to sit upright and raise his left arm at the level of the heart, with no tight clothing constricting the arm. Then an appropriately sized cuff was placed over the left arm, with the center of the bladder overlying the brachial artery, making sure the bladder encircles about 80% of the arm. The blood pressure was then measured and this procedure was repeated third, the mean calculated, and recorded. Patient were classified as
o Hypotension if their systolic blood pressure (SBP) was under 90 mmHg for adult patients (> 17 years) or under the 50th percentile of SBP according to age and height for pediatric patients
o Hypertension if the SBP was ≥ 140 mmHg in adult or > to the 95th percentile according to age and height in pediatric patients
o Normal weight if the body mass index (BMI) was between 17 to 28 kg/m2 for adults patients or between the 5th to 97th percentile for pediatric patient
o Underweight if the BMI was < 17 kg/m2 for adults or < 5th percentile for pediatric patients
o Overweight if the BMI was > 28 kg/m2 for adults or > 97th percentile for pediatric patients
• Urinary dipstick was done using fresh midstream urine after eliminated confounding factor such as fever, long standing up position, prior intense physical activity or menstruation. URIT 11 V dipstick were used. Dipstick was read after 60 seconds for proteinuria and hematuria and after 120 seconds for leucocyturia. Proteinuria, hematuria and leucocyturia was evaluated semi-quantitatively: Negative, trace, 1+, 2+ or ≥ 3+. If positive (≥ 1+), a control was done after 15 days to confirm proteinuria, hematuria or leucocyturia. Only patients who present confirm abnormalities was reevaluated after 3 months to confirm or infirm CKD. Urinary density was also noted.
• Creatinine was measured using kinetic modified Jaffe reaction.
• eGFR was classified as
o hyperfiltration if eGFR was > 130 ml/min/1.73 m2 in women and > 140 ml/min in men
o normal if eGFR was between 90 to 130-140 ml/min/1.73 m2
o low if eGFR was < 60 ml/min/1.73 m2
o patients with eGFR between 60 to 90 ml/min/1.73 m2 without urinary abnormalities were considered as normal.
• Pediatric patient was referred to patient under 20 years and adult patient to patient ≥ 20 years.
All statistical analysis was performed using Statistical Package for the Social Science (SPSS), version 20.0. Basic descriptive statistics were computed for the demographic data. Chi-square test and Fisher exact test were used for the categorical variables and t test and Mann-Whitney U test for continuous variables as appropriate. Cox proportional hazards model (setting confidence interval (CI) for Exp (B) at 95%) for predictor factor of CKD. The results were expressed as percentage (%) or number (n) for categorical variables and means ± standard deviation or median for continuous variables. The study was approved by the ethic committee of the Higher Institute of Health sciences.
A total of 107 patients were included: 81 SCA patients and 26 SCT patients. Sex repartition was comparable in both group although male seem to be more prevalent in SCA patient (62% vs. 36% p = 0.162). SCT patient were older than SCA (19.51 ± 9.97 years vs. 35.85 ± 7.86 years; p < 0.001). Most of SCA patients (n = 59, 73%) reported VOC during the last 12 months and all of them received at least one unit of blood during the last years. Only 10% (n = 8) were taking Hydroxyurea. SCD retinopathy and aseptic osteonecrosis was the main chronic complications found in SCA group. HIV was found in one SCT patients. Hypertension, diabetes, hepatitis B or C was not noted in both SCA and SCT groups (Table 1). Among SCA patients, eGFR at initiation was significant lower in older patient (< 20 years: 164.6 ± 65 ml/min/1.73 m2; 20-25 years: 167 ± 21 ml/min/1.73 m2; 25-30 years: 141 ± 8.1 ml/min/1.73 m2; > 30 years: 79.5 ± 32 ml/min/1.73 m2; p < 0.001 - Figure 1). Albuminuria was also more prevalent in older SCA patients (≤ 20 years: 4%, n = 2; 20-25 years n = 2, 15.4% and > 25 years n = 8, 47%, p = 0.004).
Table 1: Baseline characteristics of patients at the first consultation. View Table 1
CKD was diagnosed in 16 patients (SCA: n = 13 (16%) vs. SCT: n = 3 (11.5%), p = 0.418) (Table 2). Albuminuria was more prevalent in SCA (n = 12 (92.3%) vs. n = 1 (33.3%), p = 0.018) while CKD was more severe in SCA (stage 1: SCA: n = 9 (75%) vs. SCT n = 3 (100%), stage 2 or 3: SCA n = 3 (25%) vs. SCT n = 0) (Table 3). In SCA patients with CKD, eGFR was lower in older (< 25 years, eGFR = 165.8 ± 31.5 ml/min/1.73 m2 vs. ≥ 25 years 77.8 ± 31 ml/min/1.73 m2; p = 0.004 - Table 4). Prevalence of CKD was 4% (n = 2) in pediatric SCA patients and 37% in adult SCA patients.
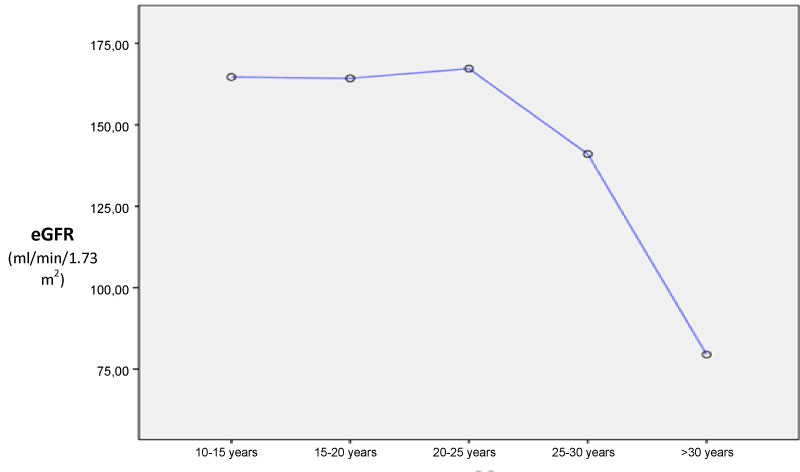 Figure 1: Means eGFR according to age at baseline.
View Figure 1
Figure 1: Means eGFR according to age at baseline.
View Figure 1
Table 2: Characteristics of patients with chronic kidney disease. View Table 2
Table 3: Urine abnormalities and CKD stage in SCA and SCT patients with CKD. View Table 3
Table 4: Comparison between SCA patient with CKD and no CKD. View Table 4
Concerning risk factor, age < 25 years was protective for both CKD and albuminuria, while SCA was a risk factor for albuminuria (Table 5, Table 6 and Table 7).
Table 5: Factors associated with chronic kidney disease in all patients. View Table 5
Table 6: Factors associated with chronic kidney disease in sickle cell anemia. View Table 6
Table 7: Factor associated with albuminuria. View Table 7
Sickle cell nephropathy is a spectrum of changes resulting from a cascade of events occurring in the kidney. This is triggered by red blood cell vascular occlusion, infarction and reperfusion injury occurring within the renal medullar, cortex and collecting system. These may present as hyperfiltration, impaired urinary concentrating ability, albuminuria, decreased eGFR and end stage kidney disease.
A previous study [11] in Cameroon found high prevalence of urinary abnormalities in SCA (albuminuria 40.3%, leucocyturia 77.8% and hematuria 13.9%). At baseline, albuminuria was found in 15% and leucocyturia in 46% of patient with SCA in our cohort. These differences could be explained by the study design as patient with vaso-occlusive crisis were excluded in our study. We also reevaluate every patient with urinary abnormality after 15 days and only patient with persistent abnormalities were considered as having baseline urinary abnormalities. Interesting, all SCA patients who had proteinuria at baseline still had proteinuria at 3 months. So dipstick should be routinely done during SCA patient follow-up and persistent proteinuria should prompt further renal investigations.
In Nigeria, macro-albuminuria was noted in 5.6% of adult SCA and micro-albuminuria in 44.4% [6]. In our cohort, among the 30 adults SCA, 33.4% (n = 10) had albuminuria and 47% of SCA > 30 years had albuminuria. In United State of America, Guasch, et al. found that 68% of adult HbSS patient had micro or macro-albuminuria and after 40 years, 40% of them have macro-albuminuria [14]. Our result suggest that prevalence of albuminuria in adult SCA is very high in our setting since only macro-albuminuria was detected. Albuminuria may also occurs more early in our SCA population, probably between 20-30 years.
Hyposthenuria was noted in 80% of our SCA patients. Urinary concentration defect in SCA begins in early infancy (6-12 months) and may account for nocturia, polyuria and enuresis in later childhood [15]. Further deterioration of the defect in urinary concentration is observed from the second decade of life due to the onset of medullary fibrosis and the loss of the collecting ducts system [15]. In patients aged 10-15 years, urinary concentrating capacity may be restored with multiple transfusions of normal RBCs. Conversely, in patients older than 15 years, the concentration defect is often irreversible and quite universal [16].
Hyperfiltration is one of the earliest manifestations of sickle nephropathy, and has been observed in children with SCA as young as 12 months of age with an age-dependent increase until the second decade of life [17]. We found that 75% of SCA patient had hyperfiltration and hyperfiltration was usual before 25 years. Haymann, et al. noted that 51% of adult SCA had hyperfiltration and 15% of them had macroalbuminuria [18]. They also find a correlation between GFR and low plasma fetal hemoglobin level, young age, and high reticulocyte count; suggesting that the pathophysiology of hyperfiltration would rather be attributable to the hemolysis-associated vasculopathy than a viscosity-vaso-occlusive process [18].
Overall CKD prevalence was 16% in SCA. In United State of America, Bodas, et al. found a CKD prevalence of 8% among children from 3-18 years-old [19] which is higher than the prevalence of 4% that we found in pediatric SCA. However, as many other studies, Bodas, et al. did not reevaluate the kidney function after 3 months. We found that 5 pediatric SCA patients had low eGFR at baseline giving a prevalence of 10% of renal dysfunction; similar to the prevalence found by Bodas. But, after 3 months, all those patients had normal eGFR. This result underline the necessity of control the renal abnormalities after 3 months to confirm CKD.
The prevalence of CKD in adult SCA patient was 37% which is similar to prevalence reported in the literature. Gosmanova, et al. in United State of America found a CKD prevalence of 28.6% in adult SCA [20] and Bolarinwa in Nigeria, a prevalence of 50% [6].
Among the 3 SCT participants with CKD of our cohort, the patient with albuminuria was HIV positive and the two other had persistent leucocyturia with normal eGFR. So, CKD in SCT in our cohort may be due to other etiologies rather than sickle cell nephropathy. Indeed, SCT have been associated with increased risk of CKD and ESRD in African American [10,21,22] especially around 60 years [21]. We found a CKD prevalence of 11.5% in SCT participant which is similar to the urban CKD prevalence of 10.9% found by Kaze, et al. in adult Cameroonian [23]. However, the SCT patient describe in our cohort are young adult with a means age of 35.85 years. In the cohort of Kaze, et al. means age was 47 years [23]. Although the size of our SCT cohort is small, this suggest SCT may be a risk factor of CKD in our setting since CKD seems to affect adult SCT before 40 years. Further studies are need to confirm it.
As noted by Bukar, et al. in Nigeria [24], we found that younger age and low systolic BP was protective factor for CKD in SCA. The relationship between sickle nephropathy and hypertension has not been well-studied. Many studies have found lower mean blood pressures and lower prevalence of hypertension among individuals with SCD compared to controls, even among those with renal disease [14,25]. Although the pathophysiology of this phenomenon is not clear, it had been hypothesized that it may be due to lower urinary concentrating ability and urinary sodium loss in individuals with SCD. Other risk factor of sickle cell nephropathy found in literature included early severe anemia, severe hemolysis, Central African Republic sickle haplotype, priapism, and hyperuricemia [26-28].
This study present limitations. Our sample size is small and may not be representative of all the Cameroonian SCD population. Albuminuria was only evaluated by dipstick and microalbuminuria was not evaluated. GFR was estimated according to CKD-EPI equation and GFR could have been overestimated in underweight participant. Another issue is that serum creatinine was measured using kinetic modified Jaffe reaction which tends to overestimate the creatinine. So, the prevalence of CKD in sickle cell disease is probably underestimated in our study. However, this study underlined the necessity of confirm kidney abnormalities at 3 months for the diagnosis of CKD.
CKD in sickle cell disease is frequent in our setting especially among young adult. It concerns SCD as well as SCT patients. Albuminuria is more frequent in homozygote patients and its prevalence increase with age. Age ≥ 25 years is associated with high risk of CKD in SCA group and albuminuria in SCD.
Authors declare no conflict of interest.