Dialysis patients may experience anemia due to functional iron deficiency, which occurs in the presence of sufficient iron stores but not enough circulating iron to provide Erythropoiesis-Stimulating Agents (ESAs) the ability to increase hematopoiesis. Current guidelines do not recommend routine administration of Intravenous (IV) iron when ferritin levels are greater than 500 ng/mL, however recent trials have shown patients receiving IV iron had further increases in Hemoglobin (Hgb) levels when ferritin was ≥ 500 ng/ml. The focus of this study is to evaluate the efficacy of IV iron therapy with epoetin alfa dosed on Hemoglobin levels for chronic Hemodialysis (HD) patients with elevated ferritin levels.
This retrospective study was conducted in patients who received chronic Hemodialysis (HD) from March 2001 to October 2009 with serum ferritin ≥ 500 ng/ml, baseline Hgb ≤ 12 g/dl, and prescribed at least one gram of ferric gluconate, iron sucrose, or iron dextran over one month. The primary objective was to evaluate the efficacy of IV iron therapies on hemodialysis patients with anemia and elevated ferritin levels receiving epoetin alfa as defined as a change in Hgb levels over a 90 day period. Secondary outcomes include changes in ferritin and iron saturation at baseline and at 90 days post IV iron therapy.
Study patients receiving Intravenous (IV) iron had an increase in the average hemoglobin by 1.4 g/dL at 90 days of therapy (p < 0.05). Iron dextran, iron sucrose, and ferric gluconate increased the average hemoglobin by 1 g/dL, 1.4 g/dL, and 1.5 g/dL, respectively. After 90 days of therapy average serum ferritin levels decreased by 75.8 ng/mL (p > 0.05). Average weekly epoetin alfa doses at 90 days differed between iron products (iron dextran group 1,867 units, iron sucrose group 2,167 units and ferric gluconate group - 3,850 units) (p > 0.05).
At 90 days of therapy, there was a statistically significant increase in average hemoglobin despite elevated baseline ferritin and low transferrin saturation levels. Intravenous iron products can provide further increases in hemoglobin in anemic chronic hemodialysis patients.
At the end of 2008 there were more than 350,000 patients receiving Hemodialysis (HD) therapy in the United States due to chronic renal insufficiency [1]. The majority of these patients have comorbid chronic anemia, exhibiting a wide range of signs and symptoms including headaches, fatigue, light-headedness, dyspnea, cognitive impairment, and chest pain [2]. There are many reasons dialysis patients experience anemia due to iron deficiency such as blood loss through dialysis and phlebotomy draws (resulting in up to 3,000 mg of iron lost per year), loss of endogenous erythropoietin production, and decreased lifespan of red blood cells making maintenance of hemoglobin concentrations within normal limits difficult [3]. Iron deficiency can be described in terms of absolute or functional deficiency. Surrogate measures of bone marrow iron stores, such as ferritin and transferrin saturation levels are used to determine if a deficiency exists. Absolute iron deficiency occurs when total iron stores become depleted and the amount of available iron is no longer adequate to meet the demands for erythropoiesis and hemoglobin synthesis. In functional deficiency iron stores are sufficient; however there is not enough circulating iron to provide erythropoietin stimulating agents the ability to increase hematopoiesis [2,3]. Therefore, dialysis patients receive iron therapy in combination with erythropoietin stimulating agents.
Although IV iron therapy in the form of high molecular weight iron dextran has been available in the United States since 1954, the first clinical prospective study on the use of IV iron was not published until 30 years later. This study concluded that IV high molecular weight iron dextran should be reserved when oral iron therapy could not be used due severe anaphylactic reactions with IV formulations. Subsequently, a black box warning was placed and a required test dose was added due to these adverse reactions. In 1991, high molecular weight iron dextran (Imferon®, Merrill Pharmaceuticals) was removed from the market with the development of low molecular weight iron but two other iron dextran products are still available (Infed® and Dexferrum®). Today, two similarly effective low molecular weight iron products are available for use in dialysis patients. Ferric gluconate, introduced in 1999, has a longer half-life than iron dextran (48 hours vs. 1 hour) and lower rates of adverse events. Iron sucrose, approved in 2000, has similar rates of adverse effects as ferric gluconate with the additional ability to directly bind to transferrin in the serum. All of these forms of IV iron are used in chronic kidney disease patients who are also receiving erythropoietin stimulation agents [3,4].
The National Kidney Foundation (NKF) clinical practice guidelines published in 2006 recommend anemia therapy with IV iron agents in HD patients receiving erythropoietin-stimulating agents. Iron is indicated for dialysis patients receiving ESA therapy with a serum ferritin < 200 ng/mL and a transferrin saturation < 20% or reticulocyte hemoglobin content > 29 pg/cell. Additionally, serum ferritin levels should be maintained between 200 and 500 ng/mL. Ferritin levels > 500 ng/mL are thought to represent excessive iron levels in dialysis patients and because there are no randomized controlled trials to compare the safety and efficacy between lower and higher ferritin target levels the guidelines do not recommend IV iron therapy for these patients [5]. These recommendations lowered the upper limit of ferritin values from 800 ng/ml from the previous guidelines [6].
After the publication of these guidelines, a randomized multi-center prospective trial, Dialysis Patients Response to IV Iron with Elevated Ferritin (DRIVE) was published [7]. The trial was conducted for 6 weeks in hemodialysis patients with anemia receiving erythropoietin stimulating agents. The authors concluded that ferric gluconate was effective in improving hemoglobin levels when ferritin levels are between 500 and 1200 ng/ml and a transferrin saturation ≤ 25%. Consequently, DRIVE-II was extended for another 6 weeks to observe the effects of a 1-gram course of ferric gluconate over a total duration of 12 weeks. The authors showed that patients receiving ferric gluconate required less epoetin and had fewer serious adverse events [8]. Pizzi, et al. examined the economic impact of DRIVE and DRIVE-II and concluded there were cost saving benefits for patients with high ferritin and low saturation levels treated with ferric gluconate and epoetin combination therapy compared to epoetin alone [9]. Despite these studies, the Kidney Disease-Improving Global Outcomes (KDIGO) guidelines published in 2012 recommended an IV iron trial if TSAT ≤ 30 and ferritin ≤ 500 ng/ml [10].
Grady Health Systems (GHS) is a 689 bed level-1 trauma center teaching hospital located in Atlanta, Georgia and housed an outpatient HD unit 1970 to 2009. The focus of this study is to evaluate the efficacy of IV iron therapy with epoetin alfa dosed at the clinicians' discretion on hemoglobin levels for chronic HD patients with elevated ferritin levels for a period of 90 days.
Patients older than 18 years of age were included if they received scheduled HD three times weekly for at least 90 days with serum ferritin ≥ 500 ng/ml, baseline hemoglobin ≤ 12 g/dl, and prescribed at least one gram of ferric iron gluconate, iron sucrose, or iron dextran over a 30 day time period. All patients had received weekly epoetin alfa treatment per routine patient care practices and was all administered subcutaneously. Study patients satisfied eligibility criteria on the day of inclusion and were excluded if prescribed IV or oral iron during the prior 90 days to avoid the potential for pre-study treatment effect. Our institution does not provide chronic Peritoneal Dialysis (PD) and no PD patients were included in the study. Patients were also excluded from the study if they received any other IV or oral iron products besides those mentioned above, had acute bleeding, an active infection or an acute inflammatory process during the study period. This study was approved by and conducted in accord with the ethical standards of our institutional review board.
This retrospective chart review utilized data collected in the dialysis outpatient clinic between March 2001 and October 2009. The primary objective was to evaluate the efficacy of IV iron in anemic chronic HD patients with elevated ferritin levels receiving epoetin. Secondary objectives are to determine if a difference in efficacy exists between IV iron products. Other objectives include changes in ferritin and TSAT values, and average weekly epoetin doses patients who received a course of IV iron therapy.
Continuous variables such hemoglobin, serum ferritin, transferrin saturation were evaluated using descriptive statistics, ANOVA and student t-test, Patient characteristics were evaluated using Chi squared. The p-value of significance for the study was p < 0.05.
This study design is a retrospective review of patient information collected from patients receiving HD treatment at GHS. Eligible patients were sequentially collected for 90 days and confidentiality was maintained using encryption and password protection. The time endpoint of 90 days was chosen to allow for the full effect of the hemoglobin and other laboratory parameters to be determined as a shorter time period. The primary outcome measure was the change in hemoglobin levels at 90 days post IV iron therapy. Secondary outcome measures include change in ferritin levels and iron saturation levels at 90 days post IV iron therapy, change in hemoglobin and average weekly epoetin alfa doses between ferric iron gluconate, iron sucrose, and iron dextran at 90 days post IV iron therapy.
During a time period of March 2001 and October 2009, 1,227 patients were evaluated from an outpatient HD database and 84 were included. From the 84 patients included there were 30 each in the ferric gluconate and iron dextran group. Twenty-four patients were in the iron sucrose group. There were no differences in patient characteristics between groups (p > 0.05) and are listed in Table 1. The majority of patients excluded from the analysis were due to not completing at least 90 days of continuous hemodialysis. The primary outcome in Figure 1, showed an average hemoglobin increase of 1.4 g/dL at 90 days of therapy with the administration of any of the intravenous iron products (p < 0.05). Furthermore, ferric gluconate increased hemoglobin to a greater extent (10.3 g/dl at baseline to 11.8 g/dl at 90 days post-treatment) when compared to iron dextran and iron sucrose (Figure 2).
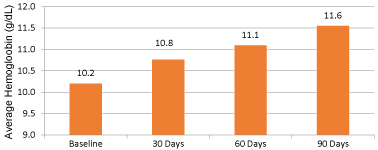 Figure 1: Difference in average hemoglobin levels at baseline to 90 days (all products). View Figure 1
Figure 1: Difference in average hemoglobin levels at baseline to 90 days (all products). View Figure 1
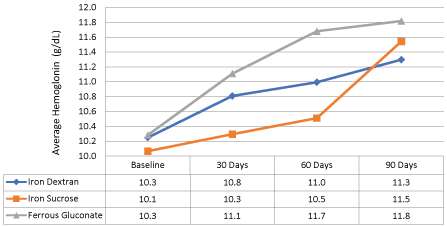 Figure 2: Difference in average hemoglobin levels between iron dextran, iron sucrose, and ferric gluconate after three months of therapy. View Figure 2
Figure 2: Difference in average hemoglobin levels between iron dextran, iron sucrose, and ferric gluconate after three months of therapy. View Figure 2
Table 1: Demographics. View Table 1
For the secondary objectives, the average ferritin level decreased 11% (696.7 ng/ml to 620.9 ng/ml, p > 0.05) while transferrin saturation remained relatively stable compared to baseline (Figure 3). Figure 4 shows the median values for ferritin between all the iron products at baseline and after three months of therapy. In all iron product groups, the median ferritin value decreased from baseline to 90 days post-therapy (p > 0.5 for each group and overall).
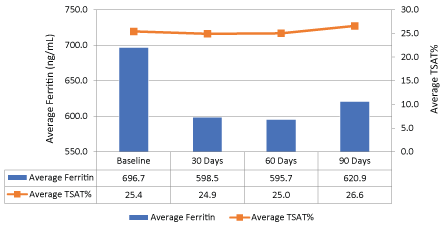 Figure 3: Difference in average ferritin and transferrin saturation at baseline to 90 days (All products). View Figure 3
Figure 3: Difference in average ferritin and transferrin saturation at baseline to 90 days (All products). View Figure 3
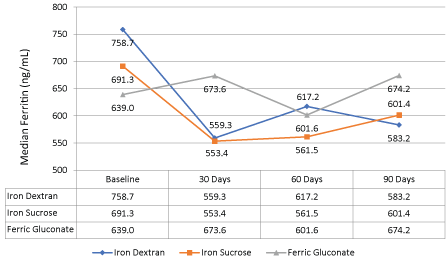 Figure 4: Difference in median ferritin between iron dextran, iron sucrose, and ferric gluconate at baseline and after three months of therapy. View Figure 4
Figure 4: Difference in median ferritin between iron dextran, iron sucrose, and ferric gluconate at baseline and after three months of therapy. View Figure 4
The NKF-KDOQI and KDIGO guidelines do not recommend IV iron when serum ferritin > 500 ng/mL [5,10], because the usefulness of serum ferritin as an iron marker for the management of anemia in chronic kidney disease is still subject to debate. There is no clear guide on how to treat patients who have a high ferritin and a low TSAT and remain anemic despite substantial epoetin dosages. Ferritin is an acute phase reactant and is often acutely elevated during states of infection or inflammation and it can be unclear whether patient's ferritin adequately reflects today body iron stores. Therefore this carries a risk of iron overload. In our study, the average ferritin level did decline during the course of the study despite IV iron therapy. This leads to the possibility that the ferritin was an acute response and the patients had a relative iron deficiency that was masked.
This retrospective review showed that anemic chronic hemodialysis patients receiving epoetin plus IV iron therapy with ferritin ≥ 500 ng/mL and normal TSAT levels may see further increases in hemoglobin and ultimately an improvement in iron deficiency anemia. Despite elevated ferritin levels at baseline, patients in our study experienced further decreases in ferritin levels after the administration of IV iron as well as increases in transferrin saturation. This supports the use of IV iron without added risk of iron overload. It was expected that increased IV iron use in this population would result in reduced epoetin doses as the finding reported with Kapoian and colleagues [8]. However, average weekly epoetin doses decreased in the ferric gluconate group but not in the iron sucrose and iron dextran therapy groups. This is likely due to the time frame of the study and the higher ferric gluconate dose at baseline.
There were several limitations to this study including that this was a retrospective chart review and any patient prescribed IV iron was considered to have received a full course of iron therapy. Pharmacy records were used to determine if doses were available in the unit but administration records were not available. This limited the ability to assess compliance of IV iron. While patients were excluded with a diagnosis of a chronic inflammatory disease, the elevated ferritin values could have be the result of an undiagnosed process not mentioned in the medical record at the time of the start of hemodialysis. In addition, unlike the DRIVE and DRIVE II studies epoetin doses in our population were not limited. This leads to the possibility that individually increased epoetin doses alone were responsible for the increases in hemoglobin seen in our retrospective analysis.
This retrospective study revealed that at 90 days of therapy, there was an overall statistically increase in average hemoglobin with all the studied intravenous iron products despite elevated baseline ferritin and low normal transferrin saturation levels. Intravenous iron products may provide further increases in hemoglobin in anemic chronic hemodialysis patients and should be considered in this select patient group.
Nothing to disclose and no potential conflicts of interest.