The development of vasculitis in renal replacement therapy is rare due to the immunological condition of these patients. Its diagnosis is often complex and invasive tests are usually required. A multidisciplinary approach is mandatory and a broad study (including lung biopsy) is necessary for its diagnosis which is crucial to support the necessity of immunosuppressive therapy in this kind of patients. We report a case of a hemodialysis patient with anti-neutrophil cytoplasmic antibody (ANCA)-negative small vessel pulmonary vasculitis de novo diagnosed two years after starting dialysis.
Lung biopsy, Vasculitis, Steroid, Hemodialysis
Currently vasculitis is a minor cause of end-stage renal disease [1]. Once on renal replacement therapy (RRT), relapse of this process is uncommon due to the immunosuppressive status of these patients [2]. Symptoms are usually associated with lung disease and although morbidity and mortality have improved in recent years they still remain high. Its diagnosis is often complex and invasive tests are usually required [3,4]. Lung biopsy (LB) is not a risk free technique but it may be necessary for the diagnosis of this entity when other studies are not conclusive [5]. We report a case of a hemodialysis patient with anti-neutrophil cytoplasmic antibody (ANCA)-negative small vessel vasculitis de novo diagnosed by LB.
A 29-year-old woman with chronic kidney disease secondary to chronic interstitial nephropathy diagnosed by a kidney biopsy, in RRT with hemodialysis for 30 months using a right jugular tunneled catheter, began tacrolimus and prednisone treatment one week before hospital admission for a scheduled related kidney donor transplant. On admission, a simple chest X-ray showed a radiopaque image in the lower lobe of the left lung, confirmed by chest computerized tomography (CT) (Figure 1). An expanded study and empirical antibiotic treatment was decided in the absence of respiratory symptoms. Mycoplasma pneumoniae MIg was positive and Candida sp was detected in induced sputum after reviewing fluconazole and specific antibiotic treatment (the patient was discharged one month later). The kidney transplant (KT) was cancelled. CT was repeated six months later when the patient was asymptomatic. A reduction of the pseudo nodular consolidation in the posterior basal segment of the inferior lobe of the left lung (LIL) was observed, but with the presence of cavitated areas inside the consolidation. In addition, new ground-glass appearance injuries were detected in the right superior lobe (RSL), in the left superior lobe (LSL), the lingula and in both lung bases. Due to presence of fever and the radiological evidence the patient was readmitted for further study.
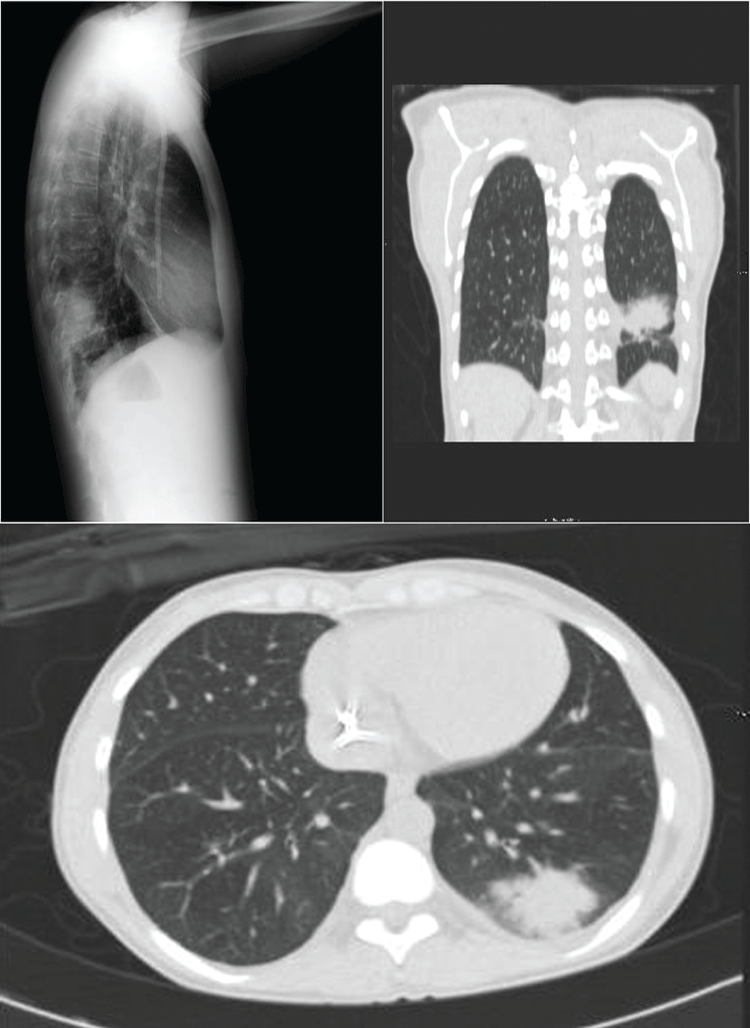 Figure 1: A) Chest computerized tomography (CT) showed radiopaque image in the lower lobe of the left lung; B) Chest computerized tomography (CT) showed ground-glass appearance injuries. View Figure 1
Figure 1: A) Chest computerized tomography (CT) showed radiopaque image in the lower lobe of the left lung; B) Chest computerized tomography (CT) showed ground-glass appearance injuries. View Figure 1
At this time, the rheumatoid factor was negative, the immunoglobulins were normal and the immunological study with ANA, anti DNA, ANCA, anti MPO, anti PR3, anti SS-A, anti-B, anti RNP, anticentromere, anti-Sm, anti-Scl-70 and anti Jo 1 was negative. Epstein Barr virus MIg, HIV serology, hepatotropic viruses, Mycoplasma pneumoniae, Legionella pneumophila and Chlamydia pneumoniae studies were negative. The polymerase chain reaction (PCR) was negative for cytomegalovirus. ACE serum levels were normal (58.3 U/l; normal range: 20-70 U/L) and the procalcitonin, galactomannan and interferon gamma assay (Quantiferon™) were negative. The tuberculin skin test, blood cultures and long-term cultures were repeatedly negative. The jugular catheter was removed and the catheter tip culture was negative. The urine cultures were negative, as was the mycobacteria test. The transthoracic echocardiography study showed no pathological findings. Two fibro-bronchoscopies were performed, the extracted samples for Nocardia and Actinomyces were negative and there was no evidence of acid-alcohol resistant bacilli. A subsequent total body CT showed no advancement of the process, ruling out involvement at other levels. A transbronchial biopsy showed preserved lining bronchial epithelium without atypia and no evidence of signs of malignancy. Bronchoalveolar lavage cytology revealed preserved respiratory cells and reactive macrophages without evidence of specific microorganisms such as Pneumocystis carinii. Neither granulomas nor necrotic areas were identified. Cytological proportional counting of 124 cells showed 72% of macrophages, 5.6% of lymphocytes, 22.5% of neutrophils and 0% of eosinophils.
Broad antibiotic treatments, including levofloxacin, meropenem, caspofungin, vancomycin, tobramycin and linezolid, was administered. Finally, in the absence of resolution of the situation LB was indicated. A videothoracoscopy was performed and the LB analysis showed abnormalities of the medium diameter vessel in the consolidation areas of the lung parenchyma. The main vascular injuries (identified) were intimal thickening, occlusion and focal recanalization of the lumen, with moderate lymphocytic infiltrates permeating the wall of the vessels. Neither fibrin on the wall, hematic extravasation, thrombus in the lumen of the affected vessels nor signs of capillaritis were observed. No fibroblast areas, polypoid lesions in the alveolar spaces, giant cells, granulomas, nor interstitial eosinophils were identified. Areas of necrosis, hemorrhage, atypical architecture or cytology were not observed. Advanced vasculitis with no specific signs in the pulmonary parenchyma was the final histological diagnosis. ANCA-negative small vessel vasculitis was diagnosed and steroid treatment was commenced, initially at a dose of 1 mg/kg/weight per day for a month, with progressive withdrawal. A minimum steroid dose (10 mg daily) was decided due to the severity of the processes and the possibility of a KT. After seventeen months of treatment in a clinically stable situation with radiological improvement, although with residual lesions, related KT was reassessed. Finally, twenty months after starting steroid treatment, a KT was performed. KT was satisfactory. Currently the patient has stable renal function and is on triple immunosuppressive therapy.
The development of autoimmune diseases is diminished in hemodialysis (HD) patients due to their attenuated immunological status. However, susceptibility to infections is also greater. It is known that relapses of vasculitis after starting HD are unusual and the development of novo processes is exceptional [1]. In our case, the patient developed an ANCA-negative small vessel vasculitis while receiving chronic HD which is an atypical occurrence.
Defining the etiology of this process is difficult because most of the studies were negative. The underlying disease of our patient (interstitial nephropathy, diagnosed by renal biopsy) does not predispose to this type of process and excludes the possibility of a recurrence of a previous illness. The emergence of de novo vasculitis is normally an exclusion finding, and its presentation made diagnosis difficult in our patient. In addition, the symptoms of this process in HD patients can be attenuated. The common kidney signs are not presented because the end stage renal failure status and the systemic symptoms are not specific. The diagnosis of this entity was based on a comprehensive study of different processes with similar radiological lung involvement. A vasculitis was suspected when considering the presence of nodules or cavitations with unclarified causes [4]. All microbiological and immunological studies were negative. Imaging studies as well as invasive studies, such as fibrobronchoscopy, did not allow the process to be defined accurately. Hence LB was mandatory although it is not risk free, but on many occasions a pathological study is necessary (to define the process) [5]. The main indications for LB are for defining atypical radiographic lesions of progressive course, distinguishing infectious processes of chronic interstitial diseases, as well as for identifying processes with specific therapeutic options with potential side effects [4,6].
LB is required in up to 30% of interstitial pulmonary processes for diagnosis [7]. However, due to its potential risks, particularly in patients with respiratory compromise or pulmonary hypertension, its indication should be very thoughtfully considered [8,9]. Sigurdsson, et al. in their retrospective study in 2009 reported that LB can modify the diagnosis in up to 73% of cases and modify the therapeutic approach in as many as 53% of the patients [10]. Currently, the results of a video thoracoscopy are compatible with an LB by open thoracotomy and the risks may be fewer [5]. Nevertheless, the indications of an LB should be a multidisciplinary decision depending on the possible complications. In our case, the LB was undertaken because of the atypical course and the clinical condition of the patient without respiratory compromise or alveolar haemorrhage during the follow-up. Finally, the LB enabled diagnosis and was decisive for specific treatment, without major complications.
The predisposition of RRT patients to suffer infective complications is widely known, and the optimal treatment is usually difficult. HD immunosuppression has been associated with increased risk of infection and poorer nutritional status. The main treatment is usually corticosteroids, isolated or associated with other immunosuppressive therapy. In our case, isolated corticosteroid therapy with subsequent gradual decrease was decided, but a minimum dose of immunosuppression was maintained because of a possible KT. After a confirmed period of stability, our patient received a KT from a related donor, with a satisfactory course and functioning to date.
The diagnosis of pulmonary vasculitis in an HD patient is rare and should only be performed after excluding infectious diseases and specific complications of the technique. The diagnosis and the treatment in dialysis are controversial and should be agreed upon in a multidisciplinary team. LB is not a complication free technique but its implementation can be crucial to reach a definitive diagnosis, to establish a prognosis, and to decide the optimal therapeutic approach.
A statement of consent from the patient has been obtained for the publication of this case report.
None.