Journal of Clinical Nephrology and Renal Care
Assessing Renal Function in Adult Myelomeningocele Patients: Correlation between Volumetric- and Creatinine-based Measurements
Vij Sarah C1*, Karim Wadih2, Luzny Patrik3, Myers Jeremy B3, Emilio Poggio1, Herts Brian2 and Wood Hadley1
1Glickman Urologic and Kidney Institute, Cleveland Clinic Foundation, Cleveland, USA
2Imaging Institute, Cleveland Clinic Foundation, Cleveland, USA
3Department of Surgery, University of Utah, Salt Lake City, USA
*Corresponding author:
Vij Sarah C, Glickman Urologic and Kidney Institute, Cleveland Clinic Foundation, 9500 Euclid Ave, Cleveland OH 44195, USA, Email: vijs@ccf.org
J Clin Nephrol Ren Care, JCNRC-2-003, (Volume 2, Issue 1), Research Article
Received: October 26, 2015: Accepted: January 27, 2016: Published: January 31, 2016
Citation: Vij SC, Wadih K, Patrik L, Myers JB, Poggio E, et al. (2016) Assessing Renal Function in Adult Myelomeningocele Patients: Correlation between Volumetric- and Creatinine-based Measurements. J Clin Nephrol Ren Care 2:003
Copyright: © 2016 Vij SC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective:Renal volume-based estimates of glomerular filtration rate (GFR) using computed tomography (CT) in renal donor populations have been described. We hypothesized that the same technology would be especially useful in myelomeningocele (MMC) patients for whom standard methods of GFR assessment are inaccurate.
Methods:13 adult subjects with MMC for whom we had CT-scans of the abdomen and pelvis with contrast were identified. 125I-iothalamate studies were available for 2 of the 13 subjects. Renal volumes were calculated from CT-scans using a fitted regression model which has been previously validated in a renal donor population. A volumetric-based GFR was then calculated. Correlation between renal volume-based GFR and Modification of Diet in Renal Disease (MDRD) and Cockcroft-Gault (C-G) GFR was calculated.
Results:Correlation between volume-based GFR and MDRD GFR was +0.76 (p = 0.003) while the correlation between volume-based GFR and C-G GFR was +0.68 (p = 0.010). Volumetric-based GFR was higher than 125I-iothalamate based estimates for the 2 patients for whom we had 125I-iothalamate scans.
Conclusions:Volumetric measurements also appear to overestimate renal function despite the classic changes in renal morphology in this population. Although renal volume based measurements of GFR correlate positively with MDRD and C-G, there is clinically significant variability in GFR calculation based on the tool used. When interpreting GFR in MMC patients, the inaccuracy of existing methods must be considered.
Introduction
Adult myelomeningocele patients represent a complex patient population managed by both pediatric and adult urologists. Impaired renal function may occur secondary to detrusor sphincter dyssynergia, poor compliance, or recurrent pyelonephritis leading to renal scarring. Therefore MMC patients often experience progressive deterioration of renal function with age. McDonnell et al. reported that 48% of adult MMC patients have renal impairment [1]. Accurately assessing the GFR in MMC patients is paramount to managing them yet current methods for determining GFR are ineffective in these patients. Many factors account for the inability of standard methods of estimating GFR. For the urologist and nephrologist, changes in the estimated GFR (eGFR) may prompt changes in management of the lower urinary tract, further exemplifying the importance of accurate eGFR assessment.
MMC patients typically have short stature with abdominal obesity and very low muscle mass, particularly in their limbs. In addition, many are non-weight bearing, reducing their muscle mass and thereby reducing creatinine generation [2]. Our primary marker for kidney function in all patients is serum creatinine, from which the GFR is then derived most commonly by the MDRD or C-G equations [3,4]. The MDR0044 equation incorporates serum creatinine, age, ethnicity and gender whereas C-G equation is based on serum creatinine and weight to determine an estimate of creatinine clearance. Serum creatinine is affected by lean muscle mass and may be abnormal in patients with low muscle mass such as MMC patients. This has been well established in elderly populations [5]. The body habitus of MMC patients also renders serum creatinine a weak predictor of GFR [5]. In addition, many of these patients undergo bladder reconstructions that incorporate bowel into urinary reservoir, which promotes re absorption of creatinine and acidosis. Quan et al. demonstrated that in MMC patients with a serum creatinine > 0.5 mg/dL, the exponential relationship between serum creatinine and GFR is completely unpredictable [6]. Cystatic C, a non-creatinine based method of estimating GFR, is under investigation [7]. Cystatin C has shown promise when compared to serum creatinine in patients with MMC, but is still not ideal [8,9]. Cystatin C measurements were not available in our study population.
Volumetric measurements of the kidneys have been used in kidney donor populations as a marker for GFR. An established radiographic protocol and derivation equations were developed and have shown excellent results in predicting GFR in this patient population [10]. Briefly, volumetric measurements are determined by measuring the volume of renal parenchyma in axial images of contrast enhanced CT-scans. We hypothesized that this unique method might serve a similar purpose in the MMC patient population. 125I-iothalamate scans are difficult to obtain with these patients due to cost and in some cases, inability to fully capture urine output reliably and therefore no gold standard comparative reference is available for the majority of the patients in our study (11 out of 13). However, we sought to assess the correlation of renal volume-based GFR with MDRD GFR and C-G GFR to determine how volumetric measurements compare to existing models.
Methods
This study is a retrospective chart review approved by the Institutional Review Board at both Cleveland Clinic Foundation and University of Utah. Laboratory and radiology studies were performed at Cleveland Clinic Foundation (10 patients) and University of Utah (3 patients). 13 MMC patients for whom radiological and serological data were available were identified from an existing internal database (2010-2014). All patients have lumbar or lumbosacral myelomeningocele and are followed by two urologists for neurogenic bladder. Mean age is 34 (range 24-47). All patients had undergone CT scan with IV contrast over the course of their urologic care. The indication for CT scan was unable to be determined based on chart review for all patients. Of the 13 patients, 2 patients had also undergone 125I-iothalamate renal clearance studies. Patients' age (years), height (cm), weight (kg), and body surface area (m2) was recorded within 1 month of the CT scan of interest. Serum creatinine was recorded at a time point within 1 month of the CT scan (prior to the IV contrast load). One radiology technologist trained in 3D image post-processing performed measurement of total renal volume as previously described in detail by Herts et al. [10]. Renal volume-based GFR was calculated using equation also described by Herts et al (renal volume-based unadjusted GFR in milliliters per minute = 70.77 - 0.444A + 0.366W + 0.2RV - 37.317SCr where A is age in years, W is weight in kilograms, EV is total renal volume in milliliters, and SCr is serum creatinine in milligrams per deciliter) [10]. The renal volume-based GFR was then adjusted for body surface area (BSA) using the method of Dubois and Dubois to determine GFR in ml/min/1.73 m2 [11]. 125I-iothalamate urinary clearance studies were performed by a dedicated laboratory and have been previously described [12]. GFR was calculated for each patient using MDRD and C-G equations previously well-established [3,4]. The original C-G equation does not correct for BSA. C-G has improved accuracy when adjusted for BSA therefore this was done to compare GFR between all four methods of GFR derivation (C-G, MDRD, 125I-iothalamte and volume-based) [13].
The Pearson correlation (r) coefficient was then calculated between renal volume-based adjusted GFR and MDRD GFR as well as between renal volume-based GFR and C-G GFR. Given that only two patients had 125I-iothalimate scans, we were unable to statistically compare renal volume-based GFR to 125I-iothalimate GFR.
Results
Patient characteristics and GFR calculations are summarized in table 1. Correlation coefficient (r) between volume-based GFR and MDRD GFR was +0.76 (p = 0.002). Correlation coefficient (r) between volume-based GFR and C-G GFR was +0.68 (p = 0.010). Correlation coefficient (r) between MDRD GFR and C-G GFR was +0.91 (p < 0.001). Volumetric-based GFR was higher than 125I-Iothalamate based estimates for the 2 patients for whom we had 125I-iothalamate scans. MDRD-based measurements overestimated the volumetric-based estimates in 6 out of 13 patients. Figure 1 shows a graphical representation of GFR by MDRD, C-G and renal volume for each patient, displaying the wide variation despite the positive correlation.
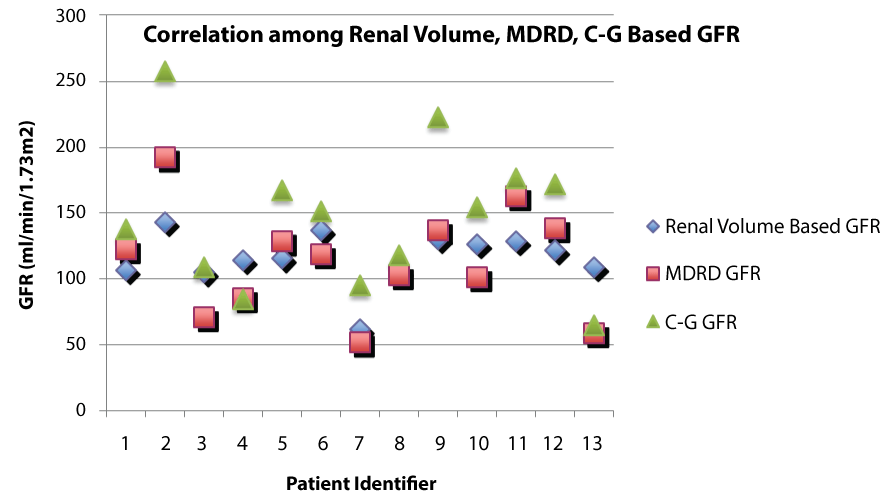
.
Figure 1: Graphical representation of GFR by renal volume, MDRD and C-G for each patient showing wide variation depending on method used.
View Figure 1
Discussion
Patients with myelomeningocele (MMC) have variable muscle mass and body composition and many have hypotonic lower extremities and/or is non-weight bearing. For this reason, measuring renal function using existing creatinine-based models is well known to be inaccurate in this population [6]. The perioperative period creates an additional challenge for MMC patients due to perioperative fluid shifts, concomitant (and often coexisting) right heart failure and respiratory disease and the need for potentially nephrotoxic drugs. Furthermore, renal function and hemodynamic assessment in this population may be further impaired due to autonomic dysreflexia, fluid resuscitation, and sometimes impaired ability to fully capture and record total urine output. Given the inaccuracy of existing models, we aimed to apply a volumetric model to this population in this study in order to better assess GFR for pre- and peri-operative management. The results of this study suggest that volumetric based measurements of GFR in MMC patients do not correlate well with existing primarily creatinine-based methods used to estimate GFR. Although we do not have the gold standard GFR for 11 out of 13 patients, we suspect from the results shown in table 1 that renal volume-based GFR likely overestimates true GFR in this population in the same way that MDRD and C-G overestimate GFR. Sagittal images of CT-scan for patient 7 are shown in figure 2 to demonstrate the radiologic appearance of the renal units in this patient population. These images show the atrophic parenchyma commonly seen in this population due to recurrent infection and poorly compliant bladders. Patient 7's GFR is estimated to be 61.88 by renal volume, 51.4 by MDRD and 94.9 by C-G.
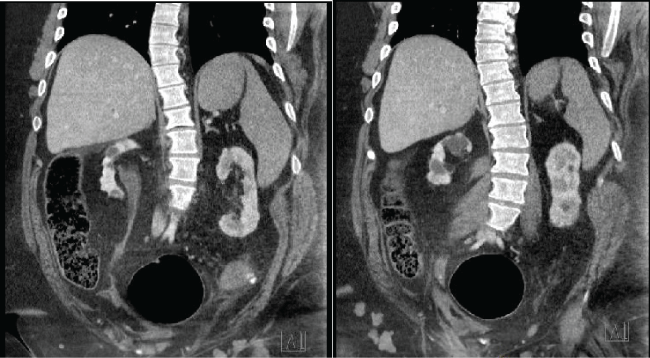
.
Figure 2: Sagittal CT scan of Patient 7 demonstrating atrophic kidneys bilaterally with minimal functional parenchyma.
View Figure 2
![]()
Table 1: Patient sex, age, absolute total renal volume (ml), serum creatinine (mg/dL), MDRD GFR (ml/min/1.73 m2), C-G GFR adjusted for BSA (ml/min/1.73 m2), 125I-iothalimate GFR (ml/min/1.73 m2).
View Table 1
125I-iothalamate and 99 mTc-diethylenetriaminepenta-acetic acid (DTPA) scans may be more accurate than estimated GFRs using serum creatinine, but are expensive, time-consuming and not immediately available at all centers. Given the accuracy of volumetric measurements in renal donor populations, we sought to identify how this method of estimating GFR correlated with other methods of estimating GFR in MMC patients. Unfortunately, this model, as validated among health renal donor patients, does not seem to accurate estimate GFR in our population. We postulate that more specific radiological techniques including both volumetric estimating and possibly elastography in combination with functional markers of renal function (e.g. Cystatin C) may hold promise in the heavily scarred, anatomically heterogenous kidneys of MMC patients.
This study has several weaknesses. Most importantly, we do not have a gold standard study to measure the volumetric measurement against in all patients. Unfortunately, 125I-iothalamate scans are not readily available and can be cost-prohibitive in many patients. Even if we had 125I-iothalamate on all these patients, we have little evidence regarding the accuracy of this method in the MMC population. The two patients for whom we did have 125I-iothalamate based GFRs, and then renal-volume based GFR was significantly higher. Defining a gold standard for this population is therefore necessary. It is clear both from the radiographic appearance of our patient population's kidneys and our knowledge of the inaccuracy of creatinine-based measurements in patients with low muscle mass, that the MDRD GFR, C-G GFR and renal-volume based GFR are inaccurate. A second weakness of this study lies in the use of BSA in a population that is wheelchair bound. BSA is also inaccurate based on presence of scoliosis and lower limb deformities. Furthermore, as Herts et al. point out, there is no accepted reference standard for renal volumes and the algorithm may include non-functional tissue such as central sinus fat [10]. Lastly, the radiation exposure associated with CT scans renders this a poor method to follow renal function long-term in these patients.
We believe that our data suggest that volumetric based measurements of GFR are inaccurate in MMC patients despite the classic changes in renal morphology in this patient population. Further research into this question with larger patient population and a gold standard measurement of GFR is essential to definitively make this conclusion. Accurate estimation of GFR remains a critical need in the perioperative management of MMC patients.
References
-
McDonnell GV, McCann JP (2000) Issues of medical management in adults with spina bifida. Childs Nerv Syst 16: 222-227.
-
Rule AD, Bailey KR, Schwartz GL, Khosla S, Lieske JC, et al. (2009) For estimating creatinine clearance measuring muscle mass gives better results than those based on demographics. Kidney Int 75: 1071-1078.
-
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, et al. (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461-470.
-
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16: 31-41.
-
Goldberg TH, Finkelstein MS (1987) Difficulties in estimating glomerular filtration rate in the elderly. Arch Intern Med 147: 1430-1433.
-
Quan A, Adams R, Ekmark E, Baum M (1997) Serum creatinine is a poor marker of glomerular filtration rate in patients with spina bifida. Dev Med Child Neurol 39: 808-810.
-
Stevens LA, Coresh J, Greene T, Levey AS (2006) Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med 354: 2473-2483.
-
Pham-Huy A, Leonard M, Lepage N, Halton J, Filler G (2003) Measuring glomerular filtration rate with cystatin C and beta-trace protein in children with spina bifida. J Urol 169: 2312-2315.
-
Morgan C, Senthilselvan A, Bamforth F, Hoskinson M, Gowrishankar M (2008) Correlation between cystatin C- and renal scan-determined glomerular filtration rate in children with spina bifida. Pediatr Nephrol 23: 329-332.
-
Herts BR, Sharma N, Lieber M, Freire M, Goldfarb DA, et al. (2009) Estimating glomerular filtration rate in kidney donors: a model constructed with renal volume measurements from donor CT scans. Radiology 252: 109-116.
-
Du Bois D, Du Bois EF (1989) A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 5: 303-311.
-
Poggio ED, Wang X, Greene T, Van Lente F, Hall PM (2005) Performance of the modification of diet in renal disease and Cockcroft-Gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol 16: 459-466.
-
Rostoker G, Andrivet P, Pham I, Griuncelli M, Adnot S (2007) A modified Cockcroft-Gault formula taking into account the body surface area gives a more accurate estimation of the glomerular filtration rate. J Nephrol 20: 576-585.





