Infections are frequent complications after liver transplantation. The impact of surgical site infections on patient outcome remains unclear. The aim of our retrospective study is to analyze the incidence and predictors of surgical site infections after liver transplant at our program and to determine their impact on patient outcome. Twenty-four (9.5%) surgical site infections were recorded among 252 liver transplants performed between January 2011 and December 2013. Among perioperative variables, re-transplantation was the only significant risk factor on univariate analysis (P = 0.015, CI 1.448-29.259), whereas age, gender, ethnicity, MELD score, donor type and cold ischemia time were not. The length of hospital stay was increased in the surgical site infection group (median 12 (5-152)) compared to the rest of the patients (median 9 (5-145)) (p = 0.032), while rejection rate was lower although not significantly different (0% versus 4.4%) (p = 0.295). Patient and graft survival at 1, 3 and 5 years were lower in the SSI group compared to non-SSI (p = 0.001 and 0.003, respectively).
In our experience, re-transplants pose higher risk for SSI compared to primary transplants. SSI increase the length of hospital stay and impact negatively on survival after liver transplantation.
Immunosuppression, Organ transplant, Post-transplant complications, Nosocomial infection, Risk factors
LT: Liver Transplantation; SSI: Surgical Site Infection; DCD: Donation after Circulatory Determination of Death; MELD: Model for End-stage Liver Disease
Liver transplantation (LT) is an established treatment for patients with acute and chronic liver failure. Currently over 6,000 liver transplants are performed every year in the US and the outcomes have improved dramatically over the last two decades compared to the early era due to the refinement of the surgical technique, more effective immunosuppression and better peri-operative care, including infection prophylaxis [1]. As a result, current 1-year survival rates approach 90% in many centers. Nevertheless, infections remain frequent complications after LT with a potential negative impact on outcomes. The most common infections after LT involve the surgical wound, the abdominal cavity, the biliary tract, the lung and the bloodstream [2,3]. In previous studies, SSI after LT have been associated with increased mortality, readmission rates and costs [4]. Recently, the emergence of multi-drug resistant bacteria has raised concern [1,5] calling for an increased awareness and updated management of SSI after LT.
The rate of surgical site infections (SSI) has been used recently by regulatory agencies as a measure of quality of care and several studies have appeared in the surgical [6,7] and transplant [8,9] literature on the incidence and impact of SSI. However, the applicability to transplant recipients of quality metrics adopted in the general surgical population is not well established. The clinical condition of transplant recipients both pre- and post-transplant is usually more complex than the general surgery population due to the effects of end-stage organ failure, poor general medical and nutritional status, frequent pre-transplant hospitalization, use of invasive equipment and vascular access, prolonged operative time and immunosuppression. As a result, LT patients are more likely to develop SSI compared to the general surgery population. Therefore, effective strategies to prevent SSI are needed in an attempt to constantly improve outcomes after LT.
The Center for Disease Control defined SSI as infections occurring within 30 days after the operation and classified them as superficial (limited to the skin and subcutaneous tissue), deep (involving the fascial and muscle layers) or organ-space infections (extending beyond fascia and muscle layer [10]. In the general surgery patient population, risk factors for SSI are the presence of coincident infections, pre-operative nares colonization with S. aureus, diabetes, cigarette smoking, obesity and the extremes of age [11]. Additionally, four operative variables were identified by the Study of Efficacy of Nosocomial Infection Control (SENIC) as independent predictors of SSI including abdominal operation, surgical time greater than 2 hours, contaminated or dirty/infected site as well as multiple co-morbidities [11]. In LT, the risk factors for SSI and the impact on outcomes are poorly defined.
The aim of our study is to review the experience at our program with SSI after LT and to analyze their incidence, predictors, and outcomes, including impact on survival.
We conducted a retrospective analysis of all adult patients (age ≥ 18 years) undergoing primary liver transplant, re-transplantor simultaneous liver-kidney transplant at our institution between 2011 and 2013. Multi-visceral transplants (liver-small bowel, liver-pancreas) were excluded. The study was approved by the Institutional Review Board.
The electronic medical records and the prospectively maintained transplant databases were reviewed to analyze the incidence, clinical presentation, microbiology, treatment and outcome of patients who experienced SSI after LT. Patients with SSI were identified by reviewing all microbiology records, operative notes, radiology reports and discharge summaries. SSI were classified according to CDC as superficial, deep and organ space [10]. In order to capture patients with prolonged hospital stay and complex post-operative course, we included in our analysis SSI occurring up to 90 days post-LT in contrast with the 30 days post-transplant interval of the original CDC definition. Other parameters analyzed in this study included donor, recipient and operative characteristics of all patients in the cohort.
All grafts were procured with standard technique from brain-dead donors or from donation after circulatory determination of death (DCD). The recipient operation included standard vena cava replacement technique without the use of veno-venous bypass. Recipients Model for End-Stage Liver Disease (MELD) score included in this analysis represents the native calculated score without exception points.
The immunosuppression protocol consisted of tacrolimus, mycophenolate mofetil and corticosteroids. In patients with SSI the immunosuppression regimen was modified as needed on an individual basis based on the risk profile. While the tacrolimus trough levels were generally maintained unchanged, mycophenolate mofetil dose was reduced or temporarily discontinued and steroid dose was tapered.
Peri-operative anti-bacterial prophylaxis consisted of ampicillin and cefoxitin for the first 48-hours post-transplant. Anti-fungal and anti-viral prophylaxis included a combination of nystatin swish-and-swallow, micafungin, fluconazole, ganciclovir and valganciclovir for 3-6 months, depending on the risk profile. The post-transplant antimicrobial therapy was modified on an individual basis considering clinical factors and speciation of microbiology results.
The length of hospital stay was calculated from the date of transplant to the date of discharge, thus excluding any duration of hospital stay pre-transplant.SSI were diagnosed according to standard criteria (see above) and treated depending on severity. Superficial SSI were managed at the bedside with wound care and antibiotics, while deep and organ space SSI were treated either with percutaneous drainage by interventional radiology or with laparotomy and open surgical drainage, in addition to antibiotics.
Continuous variables were expressed as median (range), categorical variables were expressed as percentage. The cumulative incidence of SSI, graft loss and death was estimated by using Kaplan-Meier method [12]. Univariate and multivariate analysis using the Cox proportional hazard model [13] was used to investigate the association between patient, donor and operative characteristics and development of SSI post-transplant. A similar analysis was undertaken to investigate the impact of SSI on patient and graft survival.
The demographic characteristics of 252 adult recipients of a LT performed at our program between January 2011 and December 2013 included in this study are reported in (Table 1).
Table 1: Patient characteristics. View Table 1
Among them, 23 (9%) patients developed 24 SSI at a median interval of 21 days (range7-88) post-LT. According to the CDC classification of surgical site infections (see Methods), there were 8 (33%) superficial, 4 (17%) deep and 12 (50%) organ space SSI. The clinical presentation, microbiology results and treatment course for each SSI are reported in (Table 2).
Table 2: Surgical site infections. View Table 2
Microbiology culture results were available for 21 of 24 infections. Based on culture results, the infection was polymicrobial in 13/252 (5%) and monomicrobial in 8 (3%) patients. The remaining 3 patients were treated empirically for a culture-negative symptomatic SSI based on clinical presentation.
In addition to antimicrobial therapy, 13 (5%) patients required an invasive procedure for the treatment of SSI: 7 (3%) patients underwent percutaneous drainage by interventional radiology and 6 (2%) patients required re-operation. No patient died or lost the graft due to SSI in this cohort.
Among donor, recipient and operative characteristics, re-transplantation was associated with increased risk of SSI on univariate (p = 0.015) analysis (HR = 6.51 (1.448-29.259)), while other variables such as age, gender, race, MELD score, DCD status and cold ischemia time were not significantly different between patients who experienced SSI versus not (Table 3).
Table 3: Risk factors for surgical site infections. View Table 3
The duration of hospital stay was longer in patients with SSI (median 12 days (5-152)) compared to non-SSI patients (9 days (5-145), p = 0.032). We also concluded that SSI is not a significant risk factor for rejection, which was of particular interest to us considering that patients with clinical suspicion for infection have their immunosuppression reduced (Table 4).
Table 4: Outcomes. View Table 4
There were 10/252 (4%) episodes of biopsy-proven acute cellular rejection in the present cohort during the study period of 90-days post-transplant, none of which occurred in patients with SSI.
After a median follow-up of 47 months, patient survival at 1, 3, 5 years was 84%, 80% and 79%. Survival in the SSI group was significantly different from survival in the non-SSI group at 1, 3 and 5 years. (p = 0.006). Similarly, graft survival was inferior in SSI patients (58% 58%, 58% at 1, 3, 5 years) compared to non-SSI (85%, 80%, 80%, p = 0.003) (Figure 1).
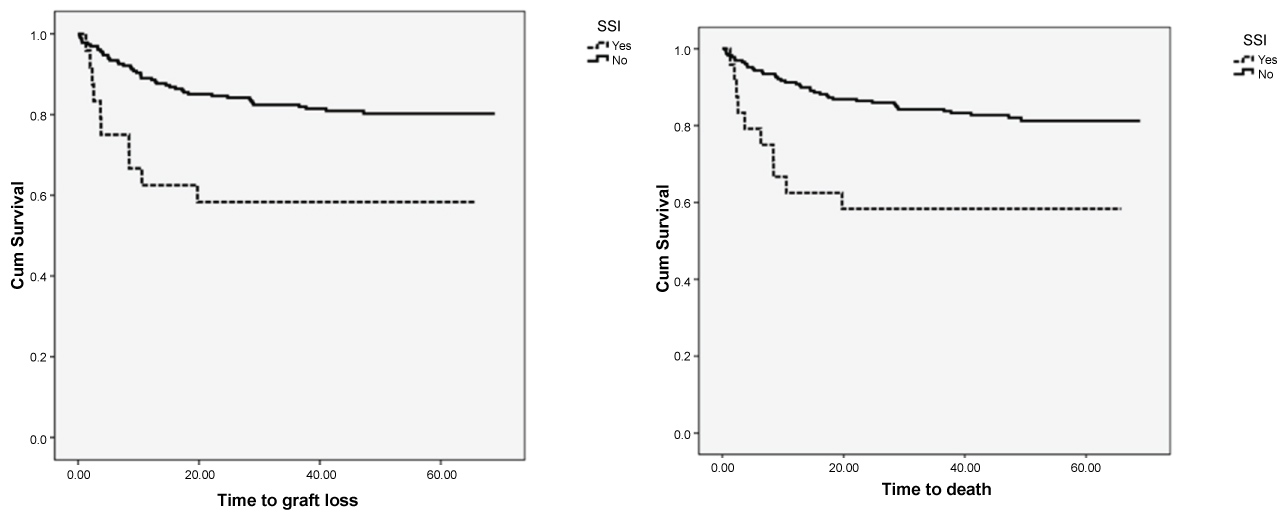 Figure 1: Patient and graft loss-kaplan meier analysis.
Figure 1: Patient and graft loss-kaplan meier analysis.
Outcomes: Survival and graft loss cumulative incidence was estimated using the Kaplan Meier Method comparing the surgical site infection (SSI) and non SSI groups and found that mortality, overall survival of both patients and grafts was significantly different between the two groups.
View Figure 1
In this retrospective study we found that the current incidence of SSI at our program is 9.5%. In our series, 24 SSI occurred in 23/252 patients after LT. One patient developed two SSI’s caused by different microorganisms: a superficial wound infection on day 18 and abdominal fluid collection on day 44 growing corynebacterium and alpha-hemolytic streptococcus.
Previous studies from other centers reported the frequency of SSI after LT between 8 and 37% and the variability is likely due to different definitions and patient selection [4,14-18].
SSI as classified by the CDC National Nosocomial Infection Surveillance system occur within 30 days of surgery. However, in our data collection we broadened the post-LT interval to 90 days after LT instead of only the first 30 days in order to capture late infections occurring in patients with prolonged post-LT course. Unlike in most elective general surgery operations, the recovery period after LT can be prolonged and complicated, occasionally extending for several weeks, especially in high MELD patients due to the advanced disease state of transplant candidates and the associate multi-organ system involvement, such as acute kidney injury and others. As a result, in such debilitated patients, SSI may occur beyond the first 30 post-operative days and would not be captured by the current definition. We believe that the expansion of the post-LT period of surveillance for SSI to 90 days more accurately reflect the severity of their disease and more reliably captures relevant infections that would have otherwise not been included. Other studies adopted this wider post-LT interval up to 90 days [19] including the very first study on this subject where the study interval was extended up to one-year post-LT [16]. The current low rate of SSI in our experience results from a combination of effective peri-operative infection prophylaxis, close patient monitoring and individualized immunosuppression management. However, their frequency remains higher compared with the incidence of SSI in the general surgery population reported between 2-4% [10] highlighting, among other factors, the impact of the immunosuppression state on the increased risk of infection.
In addition to immunosuppression, several operative characteristics, including the length of operative time, impact on the risk of SSI after LT. Previous studies emphasized that the risk of surgical site infection can be stratified based on operative time [20]. Our length of operative time for LT is usually between 4-6 hours depending on the complexity of the case (data not reported) and this duration of the operation is significantly longer compared to the threshold of 2 hours previously reported in general surgery patients [11].
Other transplant-specific risk factors for intra-abdominal infection post-LT have been identified including the type of biliary anastomosis (duct-to-duct versus roux-en-y hepatico-jejunostomy, due to the putative protective effect of preserving the native Sphincter of Oddi) and the occurrence of hepatic artery thrombosis post-LT [9,21]. Several other risk factors for SSI in LT recipients that have been reported include surgical complexity, poor nutritional status, co-morbidities, frequent prolonged pre-transplant hospital stay, invasive procedures and use of intra-vascular catheters. Some of these factors are in common with patients undergoing non-transplant liver surgery whose reported incidence of SSI varies between 7 and 27%, attributed to hypoalbuminemia, dialysis, operative time and extent of liver resection [22]. In turn, these factors lead to increased risk of colonization with multi-drug-resistant organisms [23].
Other significant findings of our study are the increased length of stay and negative impact of SSI on survival. Prior studies reported that SSI after LT are associated with significant increase in resource utilization. In a multicenter study that examined 916 LT cases between 1990-1995, patients who developed SSI (292/916) incurred an extra $160,000 in extra charges had 24 extra hospital stays on average. Further, SSI was the single most impactful factor on resource utilization when considering the cost of LT [16].
In our series the median length of stay was 4 days longer in the SSI group compared to non SSI. This has a direct impact on costs. A prior group reported that SSI patients were five times more likely to get readmitted to the hospital and twice as likely to die [15].
In addition to increasing costs, SSI also impact patient outcomes. In our experience SSI has a significant impact on patient and graft survival. This is in line with prior studies that showed that transplant patients with SSI are more likely to lose their graft, with a relative risk of graft loss or death within one year of transplant of 2.95. In the same study, the risk of graft loss or death was even greater when considering only deep or organ-space infections [4]. There is no general consensus on the most effective prevention strategy for SSI since many observations from prior studies are center-specific. According to the Cochrane Database Review from Almeida, et al. in 2015, no particular antibiotic prophylactic regimen has been identified so far to prevail over other regimens.
The strength in our study comes from a uniform protocol of single-center experience, a unified team of transplant surgeons and staff, infectious disease and transplant hepatology consultants. The detailed chart review was performed by a single investigator, ensuring all charts were reviewed in the same detailed and systematic fashion. The single-center experience is also a limitation of the study, in that it limits the overall number of patients that can be analyzed. Our results are therefore difficult to generalize to other programs. The limited sample size prevented a more meaningful multivariate analysis which would have allowed us to identify potential causal relationships between certain characteristics and SSI. The retrospective nature of this chart review may have also failed to identify certain empirically treated infections that lacked proper documentation. Finally, we did not evaluate the impact of induction immunosuppression on SSI.
In conclusion, patients undergoing re-transplantation are at a significantly increased risk for infections. SSI prolong the hospital stay and impact negatively on survival. Future studies are needed to identify independent predictors of surgical site infection and to design effective preventive strategies.
We are indebted to our Infectious Disease, Hepatology and Pharmacy colleagues and to the team of nurses and advanced care providers for excellent patient care.