This is a case report examining a urine leak and ureteric stricture following pediatric dual en-bloc renal transplantation. Despite intraoperative ureteric stenting, and post-operative nephrostomy, urinary leak continued. Both grafts grew in size from 5 cm at implantation, to 9.5 cm within 3 months. Ureteric reconstruction would expose the patient to unacceptable risk of losing both grafts, due to the close proximity of the ureters. A transplant nephrectomy of the obstructed graft was therefore undertaken. Transplant nephrectomy of a single obstructed graft is a viable option following an enbloc transplantation, where the remaining kidney can continue to provide sufficient renal replacement therapy.
En bloc, Kidney, Pediatric, Transplantation, Ureteric stricture
Ureteric stenosis is the most common non-immunological complication following renal transplantation, affecting 0.5-6% of patients [1]. This is most commonly associated with watershed ischemia of the distal ureter but may be due to BK viral infection in up to 3% of cases [2,3]. Urinary leak, usually from the vesicoureteric anastomosis, occurs in 3.5% of patients [4]. A meta-analysis [5] examining seven randomized control trials found that major urological complications were reduced by universal prophylactic ureteric stenting following renal transplantation (RR 0.24, 95% CI 0.07-0.77, p = 0.02). Ureteric stents are therefore routinely used by many surgeons [6].
We report, for the first time in the literature, the presentation and management of a pediatric dual en-bloc renal transplant complicated by urinary leak and ureteric stricture, from one graft. This was managed by nephrectomy of the affected graft, with preservation of the other.
A 26-year-old female with end-stage renal disease of unknown etiology, received an en-bloc dual renal transplant from a 1 year and 9-months-old donor after brain death (DBD). The HLA mis-match was 1-0-1. Prior to implantation, the main right renal artery, which had been injured at the retrieval, was identified and re-anastomosed on the backbench to the donor aorta. There was also a small accessory right lower polar renal artery present, in continuity with the aorta. Both kidneys were approximately 5 cm in length at implantation.
The right and left kidneys were transplanted en-bloc with the donor aorta and inferior vena cava (IVC). The donor aorta was anastomosed end-to-end onto the right internal iliac artery. The donor IVC was anastomosed end-to-side onto the right external iliac vein. The right kidney lay superio-medially in relation to the left kidney, which lay laterally (Figure 1). Both kidneys perfused well after a cold ischemic time (CIT) of 20 hours and an implantation time of 28 minutes. The donor ureters were anastomosed separately to the recipient's bladder using a modified Lich-Gregoir technique. Ureteric stents were left in-situ.
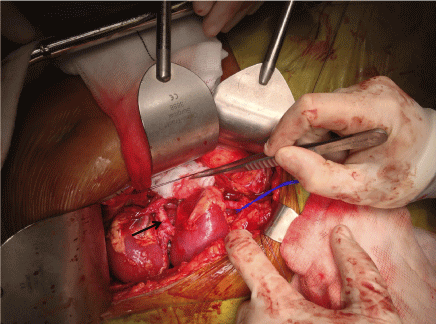 Figure 1: Intra-operative photograph: Dual en-bloc kidneys after implantation Right and left kidney measured 5.0 cm, with donor aorta (marked with black arrow) and inferior vena cava. Anastomoses to right internal iliac artery and right external iliac vein (controlled with blue sling), respectively. View Figure 1
Figure 1: Intra-operative photograph: Dual en-bloc kidneys after implantation Right and left kidney measured 5.0 cm, with donor aorta (marked with black arrow) and inferior vena cava. Anastomoses to right internal iliac artery and right external iliac vein (controlled with blue sling), respectively. View Figure 1
Post-operatively, the patient had delayed graft function and initially required hemodialysis. Urine output improved, and the patient was discharged on the 11th postoperative day with a serum creatinine of 96 μmol/L. She was readmitted three days later with clear fluid discharging through the wound, associated with pyrexia and a CRP of 212 mg/L. CT scanning demonstrated a 3.6 × 3 × 7 cm collection between the kidneys, representing a urinoma, despite adequate position of both ureteric stents (Figure 2). A urinary catheter was re-inserted, and a cystogram confirmed the presence of a urinary leak from the vesico-ureteric anastomosis related to the superior-medial kidney (Figure 3). Contrast-enhanced computerized tomography (CT) demonstrated a lack of enhancement of the lower pole of the superior medial kidney. It was therefore likely that the small right lower polar artery had thrombosed, despite perioperative anticoagulation with unfractionated heparin.
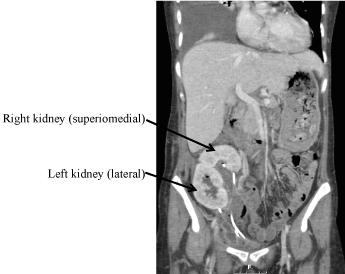 Figure 2: Computerized tomography scan with IV contrast: Dual en-bloc kidney transplants in the right iliac fossa. Transplant ureters are in close proximity with stents in situ. View Figure 2
Figure 2: Computerized tomography scan with IV contrast: Dual en-bloc kidney transplants in the right iliac fossa. Transplant ureters are in close proximity with stents in situ. View Figure 2
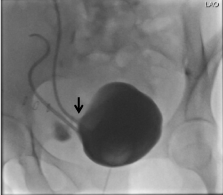 Figure 3: Cystogram: Demonstrating urine leak (marked by black arrow) from the vesico-ureteric anastomosis related to the superior graft. View Figure 3
Figure 3: Cystogram: Demonstrating urine leak (marked by black arrow) from the vesico-ureteric anastomosis related to the superior graft. View Figure 3
Despite administration of broad-spectrum antibiotics, the patient developed worsening sepsis and acute kidney injury, with CRP rising to 373 mg/L and serum creatinine rising to 120 μmol/L. A further CT scan showed two pelvic collections of 9 × 6 cm and 7.0 by 5.5 cm. These represented infected urinomas, and a percutaneous drain was inserted into the larger of these.
A repeat cystogram 7 days later showed no evidence of ongoing urine leak and so the urinary catheter was removed. The patient remained well, with a serum creatinine in the range of 69.8-90.2 μmol/L, in the following few weeks. The ureteric stents were removed 4 weeks after the second cystogram. Following stent removal, the patient developed a sharp rise in serum creatinine to 150 μmol/L. An ultrasound indicated the superio-medial kidney was hydronephrotic. A MAG-3 renogram demonstrated obstruction of this graft. The patient had also developed BK viremia (7000 copies/ml) at this time.
A nephrostomy was inserted into the obstructed graft. The nephrostogram demonstrated distal ureteric obstruction and an attempt at antegrade ureteric stent insertion failed because the ureter was completely occluded at the level of the sacroiliac joint (Figure 4). The nephrostogram was left in-situ while two therapeutic options were discussed with the patient:
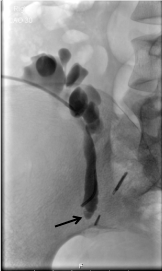 Figure 4: Nephrostogram of the superior graft: Demonstrating obstruction at the level of the sacro-iliac joint (marked by black arrow). View Figure 4
Figure 4: Nephrostogram of the superior graft: Demonstrating obstruction at the level of the sacro-iliac joint (marked by black arrow). View Figure 4
1. Reconstruction of the obstructed graft using the right native ureter.
2. Transplant nephrectomy of the obstructed graft, leaving the inferior graft in situ.
Following discussions within the multi-disciplinary team, it was decided that reconstruction of the ureter would expose the patient to unacceptable risk of losing both grafts, due to the close proximity of the two transplant ureters. In contrast, transplant nephrectomy of the hydronephrotic graft was expected to provide the patient with sufficient renal function alone, as the non-obstructed kidney had now grown to 9.5 cm in length.
A transplant nephrectomy of the obstructed graft was undertaken, with no significant complications and a hospital stay of 4 days. Following this procedure, serum creatinine was 90 μmol/L.
This case report demonstrates urine leak and ureteric stricture in a dual en bloc renal transplantation. This poses unique challenges, given the proximity of the grafts and ureters. The mainstay of treatment remains the same for all causes of ureteric stricture: Radiological or surgical. Transplant nephrostomy with antegrade ureteric stenting is a common initial treatment. Surgical options include excision of the stenotic segment with reimplantation, with or without a psoas hitch of the bladder, a Boari flap, or ureteric reconstruction using native ureter. Extra-anatomical stenting has also been described [7]. However, these options may put the second transplant ureter and graft at risk of damage. Transplant nephrectomy of a single obstructed graft is an option following an en-bloc transplant [8], where the remaining kidney can continue to provide sufficient renal replacement therapy, as was successfully demonstrated in this case.
None.
The authors declare that they have no conflict of interest.