The infectious aneurysm in kidney transplant carriers constitutes 1-3% of all the reported aneurysms. In this population the frequency of infectious complications is high due to the presence of over immunosuppression episodes.
A 27-years-old female with end stage kidney disease, received a deceased donor kidney transplant, the induction immunosuppression consisted in polyclonal anti-lymphocyte therapy. The maintenance therapy included plus to the immunosuppressive drugs, valganciclovir as prophylaxis of CMV. Later the patient developed abdominal pain and progressive leucopenia; right pyosalpinx was diagnosed, the salpingectomy was performed. The condition of the patient got worst and a second survey was done, finding an infectious aneurysm of the external iliac artery, with the necessity of a vascular reconstruction
Polyclonal anti-lymphocyte induction therapies are well known concerning the prognosis of the graft benefit, but also for their potential to generate infections. All immunosuppressive drugs give an increased risk for the development of infections, such as the mycotic aneurysm.
Immunosuppression should be monitored closely to avoid the development of over immunosuppression which increases the risk of infectious features such as the infectious aneurysm. This condition should be considered as a possible complication of renal transplant carriers, associated with a high mortality rate and I high chance of graft lost.
The infectious aneurysm, also called mycotic aneurysm, it's an infection of the total thickness of the arterial wall, causing an irreversible dilation of the vessel, weakening and destruction of it. These lesions may be lethal without an opportune diagnosis [1].
Mycotic aneurysm in transplant cases constitutes only 1-3% of all the reported aneurysms. The adjective of mycotic aneurysm was established in 1885, from its original description by Osler, due to its fungal shape, but not because of the etiology [2], the most frequently isolated agents are Salmonella sp. and S. aureus; although in immunocompromised patients can be any opportunistic germ [1]. In transplant carriers the frequency of serious infectious complications is high, especially in kidney transplant according to the need of permanent maintenance immunosuppression, and the high incidence of over immunosuppression episodes.
Thus we present a case of a mycotic aneurysm in a kidney transplant carrier with an episode of over immunosuppression.
A 27-year-old female with history of end stage kidney disease, of unknown etiology, with 2 years of progression. She received a deceased donor kidney transplant, with ideal criteria, the induction immunosuppression consisted in low dose of polyclonal anti-lymphocyte therapy, Mycophenolate Mofetil (MMF), and methylprednisolone, doses according to our transplant protocol. The vascular reconstruction of the renal graft was performed in the right external iliac vessels and an extravesical ureteral implant was done, total cold ischemia time was 12 hours. With a serum level of creatinine of 1.0 mg/ml, the patient was discharged; the maintenance immunosuppression with tacrolimus, MMF, and prednisone doses according to our protocol. Antibiotics administered as prophylactic treatment were sulfamethoxazole trimethoprim, nitrofurantoin and valganciclovir. The follow-up of the patient was every month after the first month, without any complications (Table 1).
Table 1: Protocol for prophylaxis and immunosuppression in kidney transplant recipients of the Health Institute of the State of Mexico. View Table 1
In the fifth month after the transplant the patient came to the emergency department with intermittent pain referred in the renal graft, with the following results of laboratory tests: creatinine 1.0 mg/dl, leucocytes 2100/mm3, total neutrophils 220 cells/μL, hemoglobin 14 g/dl, platelets 235,000/mm3, normal urine test; no other relevant data. MMF dose was reduced to 1 g, valganciclovir was suspended and the patient was readmitted to complement the leucopenia survey.
Graft Doppler ultrasound was performed finding an adequate perfusion. Within the next 24 hours the pain persisted, developed fever up to 38 degrees, and the leucopenia got worst (leucocytes of 1000/mm3, with neutrophils < 100 cells/μL), MMF was suspended, samples for blood cultures were processed. We started ceftriaxone, and performed an abdominal CT scan with contrast, finding a cystic image attached to the right ovary, the renal graft seemed to be normal, without any anomaly in the renal vessels. Therefore we proceed with laparoscopically approach, with the following findings: right pyosalpinx, attached to the graft intraperitoneally, the cystic image corresponded to an abscess, both ovaries with normal appearance (Figure 1). Right salpingectomy was done, and additionally to the ceftriaxone we administered doxycycline. After the surgery the patient had clinical improvement, remission of the pain, and improvement of the leucopenia, she was discharged after 4 days, with cephalexine, doxycycline, without valganciclovir and MMF; white blood count had slightly improved, with leucocytes of 3000/mm3, and neutrophils 350 cells/μL. Blood cultures yielded negative results for any microorganisms.
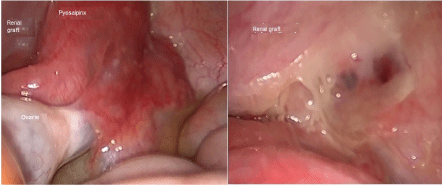 Figure 1: Left: Pyosalpinx attached to the renal graft; Right: Intraperitoneal view of the renal graft after the salpingectomy. View Figure 1
Figure 1: Left: Pyosalpinx attached to the renal graft; Right: Intraperitoneal view of the renal graft after the salpingectomy. View Figure 1
After 72 hours the patient was readmitted due to severe abdominal pain, constant, fever and malaise. Laboratory tests with 800 leukocytes, hemoglobin of 10 g/dl, platelets 235,000 and preserved renal function (creatinine 1.0 mg/dl). CT scan was updated finding a pelvic tumor adjacent to the renal graft of 5 × 8 × 6 cm, with enhancement in the arterial phase, establishing the diagnosis of renal graft aneurysm, which involved the right external iliac artery (Figure 2 and Figure 3).
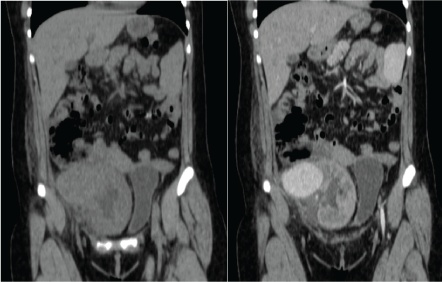 Figure 2: Left: CT scan showing a tumor attached to the renal graft; Right: CT with contrast showing and enhancement of the tumor. View Figure 2
Figure 2: Left: CT scan showing a tumor attached to the renal graft; Right: CT with contrast showing and enhancement of the tumor. View Figure 2
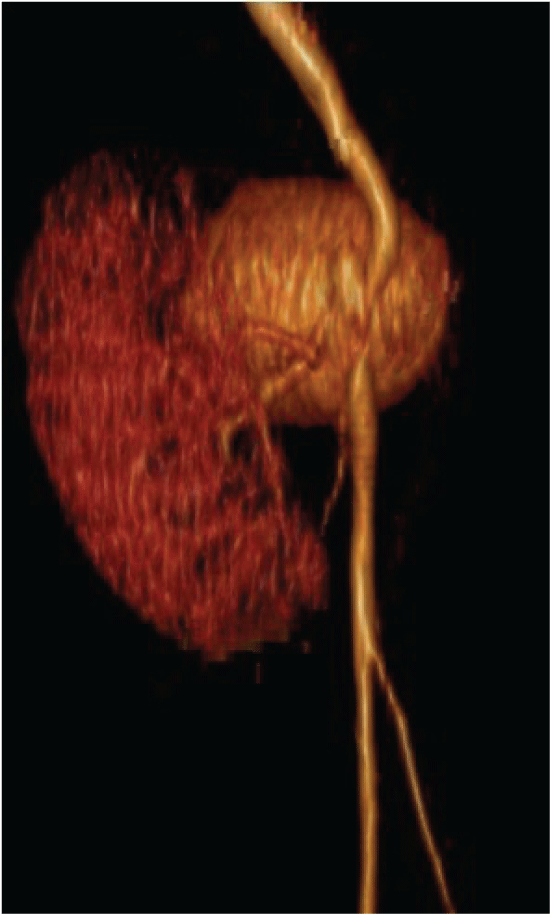 Figure 3: Tridimensional reconstruction of the aneurysm with a medial displacement of the renal graft View Figure 3
Figure 3: Tridimensional reconstruction of the aneurysm with a medial displacement of the renal graft View Figure 3
For the definitive management the aneurysm was resected, the right iliac artery was replaced with a prosthetic vascular graft, the anastomosis of the artery of the renal graft was attached it, with an adequate intraoperative reperfusion, however within 24 hours with the Doppler ultrasound arterial thrombosis was found and the renal graft was removed.
Tissue culture from the resected aneurysm showed Candida albicans; nevertheless the blood cultures that were performed during the onset of symptoms, and the readmission, resulted in negative findings for any microorganisms. The pathology report of the explanted graft showed a class III antibody-mediated rejection, negative C4D, associated with pale infarcts and focal thrombotic microangiopathy.
Polyclonal anti-lymphocyte induction therapies, are well known concerning the prognosis of the graft benefit [3], but also for their potential to generate infections, which gives the recipients, regardless of the natural risk of CMV, a high risk for reactivation or for primary infection, with a variable risk of developing the disease without the appropriate prophylaxis with valganciclovir [4,5].
In a prospective analysis by Brum, et al. searching for risk factors for the development of leucopenia, they found that the use of valganciclovir and MMF, the origin of the graft (higher risk in deceased donor) and the start of the prophylaxis in the first post transplant day were the most related factors [6,7]. The main side effect of valganciclovir is leucopenia, which occurs in 10-55% and severe neutropenia in 7-37%, another event reported is the bone marrow dysfunction [6].`
The bone marrow dysfunction evolves to aplastic anemia, when the dose of valganciclovir is not adjusted according to renal clearance; this dysfunction is observed between the days 18-20 and persisted despite stopping the medication during 7-10 days. When the renal function is decreased, the half-life of the drug can increase up to 13 times. It has not been reported to MMF itself cause bone marrow toxicity with such severity; however, in cases of aplastic anemia induced by valganciclovir (confirmed by biopsy) it has been observed increase in serum free fraction of MMF, which could explain the synergistic effect for the development of leucopenia [5,6].
With the immunosuppressant as a predisposing factor a decision should be made regarding its continuation for the preservation of the graft or its suspension when the situation becomes life threatening [8].
The clinical feature of the infectious aneurysm it's inaccurate, which may include intermittent pain in the aneurysm site, malaise and fever, and finally the development of sepsis with poor response to the medical treatment. The pain occurs in up to 76% of cases and is related to its location and the speed growth [9,10]. Rupture is reported in 45% and fistula formation only in 18% of cases; the 30-day mortality reported in Lopes, et al. series was 36%, associated to rupture and fulminant sepsis [1].
The CT scan is the study of choice in the diagnosis of infectious aneurysms, as it has a sensitivity of 92-96% and a specificity of 93-100%; plus the aneurysm de novo detection, the CT scan may help to determining the growth rate, changes in morphology, the discovery of synchronous lesions, the presence of gas, the peripheral tissue edema, among others [1,9].
A key part of the management is the identification of the causative agent, which is achieved by blood cultures (with the development of the causal agent in 50-70% of cases) and in the resected aneurysm tissue cultures (with the development of 15% of cases). The absence of growth of any microorganism in the cultures do not rule out the infectious origin of the aneurysm [9].
The infectious etiologic agent may be originated from an endovascular source [8]; from a distant source that travels via lymphatic or hematogenous; and/or by direct contact with an organ or vessel; then the microorganism is established in a previously injured endothelium. In this case direct contact of the renal graft hilum with the infected oviduct through the peritoneum may be a likely route for entry of the infectious agent in a previous damaged endothelium thus the final report for the explanted kidney reported rejection [1].
The initial empiric antibiotic treatment should be considering for gram positive coverage as these are the agents that have been mostly isolated. The fungal infections occur only in 1% of cases, anywise this cause should be suspected in the presence of inmunosupresión [10].
There are no guidelines for the management of the infectious aneurysms; however both antibiotic management and resection of the aneurysm with its corresponding reconstruction for the affected vessel must be considered [1]. The suggestion in the duration of the medical treatment is 3-6 months; plus monitoring using markers of inflammation, blood cultures and tomographic control [9,11].
The medical management without surgery has a mortality of 50%, since antibiotics limit sepsis but do not prevent rupture of the vessel. Surgical management planning should include the monitoring of any distant septic source [1,12]. A treatment modality is the excision of the infectious aneurysm and placement of prosthetic material preferably medicated; another modality is the endovascular management [12].
Leonardou, et al. reported a 5 case series of mycotic aneurysm in kidney transplant recipients, using a combination of antibiotic therapy, surgery and interventional procedures, with a 100% survival of the patient, but graft lost in all cases [12].
Immunosuppression should be monitored closely to avoid the development of over immunosuppression and/or leucopenia which increase the risk of infectious features that may require medical or surgical management such as the infectious aneurysm. This condition should be considered as a possible complication of renal transplant carriers, associated with a high mortality rate if it's not treated, and I high chance of graft lost.
Both authors whose names are listed below have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.