International Journal of Transplantation Research and Medicine
Infection after Pediatric Living Related Liver Transplantation: Timing, Types and Risk Factors
Behairy El-Sayed Behairy1, Hatem Abdel-Sattar Konsowa1, Haidy Mohammed Zakaria2, Osama Hegazy Abd-Elsalam3 and Mostafa Mohamed Sira1*
1Department of Pediatric Hepatology, National Liver Institute, Menofiya University, Egypt
2Department of Pediatrics, Quesna Central Hospital, Egypt
3Department of Hepatobiliary Surgery, National Liver Institute, Menofiya University, Egypt
*Corresponding author:
Mostafa M Sira, MD, Department of Pediatric Hepatology, National Liver Institute, Menofiya University, 32511 Shebin El-koom, Menofiya, Egypt, Tel: +2-048-222-2740, Fax: +2-048-223-4586, E-mail: msira@liver-eg.org
Int J Transplant Res Med,
IJTRM-1-012, (Volume 1, Issue 3),
Research Article
Received: October 03, 2015: Accepted: November 14, 2015: Published: November 16, 2015
Citation: Behairy BES, Konsowa HAS, Zakaria HM, Elsalam OHA, Sira MM (2015) Infection after Pediatric Living Related Liver Transplantation: Timing, Types and Risk Factors. Int J Transplant Res Med 1:012
Copyright: © 2015 Behairy BES, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:Over the last several decades, the field of solid organ transplantation (SOT) science and practice has advanced significantly, only to be continually challenged by the risks for infection in SOT recipients.
Aim: We aimed to study the risk factors for post-transplant infection.
Methods: The study was a retrospective cohort. It included 27 pediatric patients underwent liver transplantation (LTx). All patients' records were reviewed. A wide range of potential risk factors for infection and post-transplant complications were recorded. Follow-up data were collected for 1.5 years post-transplant, every infection attack during this period were subjected to detailed risk analysis.
Results: The first month post-LTx encountered 50% of infections. Older age and higher body mass index of the donor, prolonged short ischemia time, anhepatic phase, roux-en-y biliary anastomosis and intensive care unit (ICU) stay duration were associated with higher risk of infection. Bacterial and fungal organisms were predominant in the 1st month post LTx, while viral and protozoal organisms were predominant thereafter. Rejection is one of the common complications that initially misdiagnosed as infection. Aspartate transaminase, alanine transaminase, alkaline phosphatase and gamma-glutamyl transpeptidase at cutoff levels of 101, 101, 433 and 231 IU/L respectively were significant discriminators between infection and rejection, with higher levels in the latter.
Conclusion: Infection is a significant cause of morbidity after LTx. Inappropriate donor selection, surgical complications and ICU stay duration were associated with higher risk of infection. The 1st month post LTx was the most critical period; infections predominant during this period were mainly of the bacterial and fungal types. Transaminases and biliary enzymes, but not FK trough level, were significant discriminators between infection and rejection with an overall 88.4% accuracy.
Keywords
Infection, Liver transplantation, Pediatrics, Rejection
Introduction
End-stage liver disease in children presents a challenging array of medical and psychosocial problems for the health care delivery team [1]. In such cases, liver transplantation (LTx) might become the only definitive treatment. In previous decades, pediatric LTx has become a state-of-the-art operation with excellent success, limited mortality and offers the opportunity for a healthier life. Graft and patient survival have continued to improve as a result of improvements in medical, surgical and anesthetic management, organ availability, immunosuppression, and identification and treatment of postoperative complications. Moreover, in some circumstances a smaller probability of long-term success may be a very worthwhile outcome for some children and their families [2].
Infections remain a significant cause of morbidity in patients undergoing LTx [3]. After solid organ transplantation, there are three time periods when specific infections are likely to occur. During the 1st period, the month immediately after LTx, infections are typically of nosocomial origin. Most infections are related to surgical issues and post-operative complications. Bacterial and candidal wound infections predominate during this period [4].
The next period encompasses the 2nd to 6th post-transplant months. During this time, infections from opportunistic organisms predominate as a result of cumulative immunosuppression [5]. From approximately the 7th to 12th post-transplant months and beyond, most recipients acquire infections similar to the infections acquired by patients who have not received transplants [6].
Infection in the LTx patient is diagnosed in the same way as in the non-transplanted population. However, it tends to be laborious work-up due to the wide differential diagnosis and the attenuation of clinical manifestations because of the immunosuppressive medications [7].
The aim of this work was to study the risk factors for post transplant infection in pediatric living donor liver transplantation (LDLT).
Patients and Methods
Study population
Twenty-seven pediatric patients who underwent LDLT at the National Liver Institute, Menoufiya University, between April 2003 and June 2011 were enrolled in this study. They were 13 (48.1%) males and 14 (51.9%) females. Their age ranged from 0.7 to 17.8 years (mean 5.3 ± 5.4). Etiological diagnoses of the transplanted patients were 11 (40.7%) with biliary atresia (BA), 5 (18.5%) with familial progressive intrahepatic cholestasis (PFIC), 3 (11.1%) with venous outflow obstruction, 2 (7.4%) with chronic hepatitis C virus (HCV), 2 (7.4%) with congenital hepatic fibrosis, two (7.4%) with hepatic malignancy, one (3.7%) with Wilson disease and one (3.7%) with cryptogenic liver cirrhosis. One of the 27 patients died on the 2nd post-operative day from primary graft non-function, so he was not included in the analysis of infection attacks.
Data collection
The study was a retrospective cohort. All patients' records were reviewed, including the entire medical and nursing staff records, the medical prescriptions, laboratory and radiological reports. Events such as infections, allograft rejection and post-transplant complications were listed. Follow-up data were collected for 1.5 years post-transplant. This time period was chosen for the purposes of the detailed risk analyses for infections as they represent the peak period for the development of different types of infections [8]. A wide range of potential pre-LTx and post-LTx recipient risk factors, donor and surgical risk factors (Table 1) were selected to be evaluated in a risk analysis for the occurrence of infection.
Routine ultrasound and laboratory investigations as complete blood count, liver function tests, renal function tests and viral markers were performed pre-LTx, two weeks post-LTx and upon every infection attack.
The LTx recipient has been suspected to have an infection attack if there were clinically evident symptoms and signs of infection as fever, cough, diarrhea or painful micturition with evident elevated total leukocyte count, elevated liver enzymes or elevated C-reactive protein (CRP). Diagnosis of infection was confirmed by positive culture of specimen taken from the infection site or positive viral markers. During the regular follow-up visits, patients were routinely checked for CRP and complete blood count. Those with elevated total leukocytes count and/or positive CRP were scheduled for blood, urine and stool cultures. Those with positive culture results in absence of clinical manifestations were defined as having subclinical infection. The LTx recipient has been suspected to have a rejection attack if he developed fever, malaise, right upper quadrant and right flank pain, jaundice, clay-colored stool, hepatomegaly and increased ascites accumulation after exclusion of other causes (as infection), associated with elevated serum transaminases, alkaline phosphatase (ALKP) and bilirubin levels. Rejection was suspected also if no satisfactory response to culture guided antibiotic and antifungal therapy or specific antiviral therapy occurred. Diagnosis was confirmed by liver biopsy.
One of the shortcomings of our study is that some data were missing from the patients' files and some surgical and ICU techniques have changed throughout the period of the study. Another limitation is that a number of risk factors couldn't be examined based on the available records e.g. preoperative antiseptic showering, the type of surgical staff hand/forearm antisepsis performed, intra-operative glove perforation, ward environment and infection status of the patients' relative.
Statistical methods
Qualitative data were expressed as frequency and percentage. Quantitative data were shown as mean ± standard deviation (SD). Significance was tested by χ2 for qualitative variables. Student t-test or the Mann Whitney U tests were used to compare means according to the nature of the data. Multivariate analysis was done to look for an effect of one or more independent variables on several dependent variables. Correlations were tested by Spermann's test. The clinical performance of aspartate transaminase (AST), alanine transaminase (ALT), gamma-glutamyl transpeptidase (GGT) and ALKP were assessed by the receiver operating characteristic (ROC) curves. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were presented as percentages. P (probability) value were considered significant if it was < 0.05. Data were analyzed using the statistical package for the social sciences (SPSS), version 18.0, (SPSS Inc., Chicago, Illinois, USA).
Results
Infection rate
Twenty-six patients developed 62 infection attacks during the whole follow-up period (1.5 years) after LTx (2.38 attack/patient) of which 40.3% were subclinical and 32%, were chest infections (Table 2). Six (9.67%) attacks were serious infections that led to death (5 were pneumonia and 1 was surgical wound sepsis). Fifty percent of the 62 infection attacks occurred in the 1st month post-LTx and 50% occurred in the remaining follow-up period (Table 2).
![]()
Table 1: Potential risk factors for post-LTx infection.
View Table 1
![]()
Table 2: Clinical and microbial types of infections in relation to the time-course post-LTx.
View Table 2
Of the 62 infection attacks, 50 were proved by culture and viral markers, of which 60%, 28% and 2% were isolated bacterial, viral and protozoal infections respectively while 10% were mixed infections (Table 2). Moreover, bacterial and fungal organisms were significantly predominant in the 1st month post-LTx, while viruses and protozoa were predominant after the 1st month (P < 0.05) (Table 2 and Figure 1). The frequently observed bacterial organisms in the 1st month were Klepsilla (17.1%) and Staphylococcus aureus (17.1%), and fungal strains were candida albicans (5.7%). The commonest viruses after the 1st month were cytomegalovirus (CMV) (23.8%) and Epstein-Barr viruses (EBV) (19%) (Table 3).
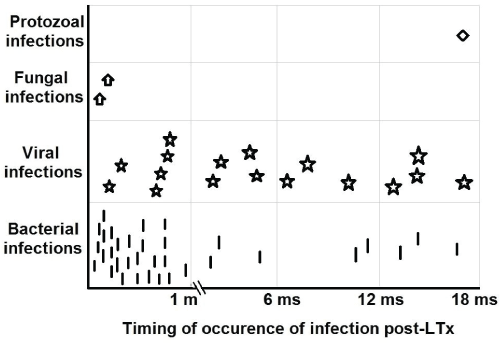
.
Figure 1: Microbial types of the infection in relation to the time-course post-transplantation.
View Figure 1
![]()
Table 3: Causative organisms of the infection attacks in relation to the time-course post-LTx.
View Table 3
Risk factors for infection
In this study there was a positive correlation between pediatric end-stage liver disease (PELD)/Model for end-stage liver disease (MELD) scores [9] and the number of infection attacks in the 1st month post-LTx (r = 0.459, P = 0.036). In the univariate analysis model, older age and higher body mass index (BMI) of the donors were related to post-LTx infection in the recipients during the 1st month (P < 0.05), while there was no significant relation to the other studied parameters. In the multivariate analysis model, the donor age and BMI showed no significance. The higher incidence of infections were in patients who began their immunosuppressive regimen the day just before LTx (63.7%), while there was no significant difference as regard the starting dose of immunosuppressant (P > 0.05) (Table 4). We found that the period of intensive care unit (ICU) stay encompasses 88.9% of infection attacks in the 1st month post-LTx and 77.4% of the infection attacks during the total period of post-operative hospitalization, while there was no statistical significance regarding the other studied parameters.
![]()
Table 4: Comparison of demographic, pre-operative, operative, post-operative and donor data between patient with and those without infection during the first post-operative month.
View Table 4
Discrimination between infection and rejection
Rejection occurred in 9/26 (34.6%) of recipients during the whole follow-up period. Acute rejection accounted for 56.3% of total rejection attacks, while chronic rejection occurred in 43.7% and none suffered hyper-acute rejection. In the univariate analysis model, the mean levels of AST, ALT, ALKP and GGT were significantly higher in patients with rejection than in patients with infection (P < 0.05), while total bilirubin and FKL were comparable in both conditions (Table 5). In the multivariate analysis model, the ALT showed no significance. AST, ALT, ALKP and GGT at cutoff levels of 101, 101, 433 and 231 IU/L, respectively, were able to discriminate between rejection and infection attacks. The highest accuracy were for ALKP (84.4%) followed by the GGT (80.2%) (Figure 2). In addition, combining these parameters together had 81.3% sensitivity, 95.5% specificity, 86.6% PPV and 95.5% NPV with the accuracy increased up to 88.4%.
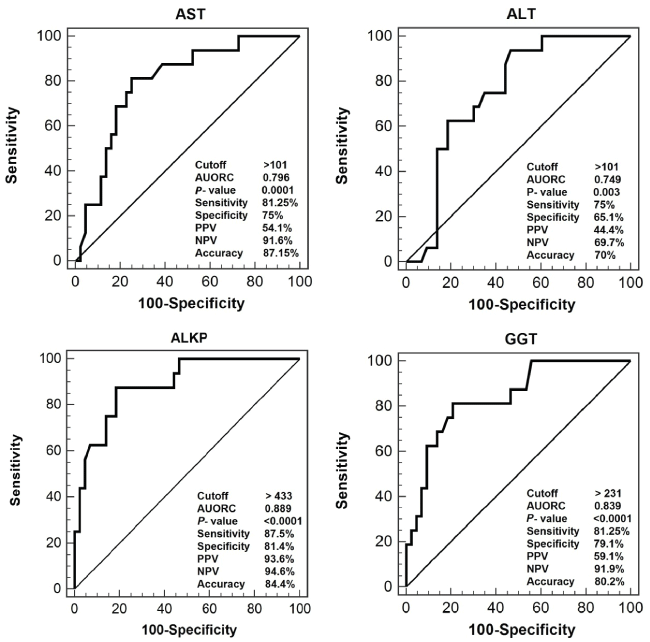
.
Figure 2: Performance of transaminases and biliary enzymes in discriminating patients with infection and those with rejection.
View Figure 2
![]()
Table 5: Transaminases, biliary enzymes, bilirubin and FKL levels in patients with infection compared to those with rejection.
View Table 5
Discussion
Liver transplantation is an established line of therapy for irreversible acute and chronic liver disease. It has dramatically changed the prognosis of children dying of liver failure [10]. The current study revealed that, BA was the commonest indication for LTx (40.7%) followed by PFIC (18.5%). In agreement with our results Engelmann et al. [11] reported that BA accounts for at least 50% of all liver transplants in children.
As a threat for the success of LTx, infection was the interest of the hepatologists. In the current study we aimed to shed the light on the frequency and risk factors related to post-LTx infection.
The 1st month post-LTx is considered the most critical period in the post-transplant life. Despite the short duration, it encountered nearly 50% of infection attacks. Allover the study period, 62 infection attacks have occurred (2.38 attack/patient), of which, only 6 (9.67%) were serious infections that led to death (5 of these were pneumonia and 1 was surgical wound sepsis). Subclinical infection accounted for 40.3% of the infection attacks that occurred in 18 months pos-LTx, followed by chest infections (32%). This means that a large percentage of patients suffering infection may not present clinically. For that, close follow up and the use of sensitive monitors of infections are very critical in post-LTx patients.
Bacterial and fungal organisms were predominant in the 1st month post-LTx, while viral and protozoal organisms were predominant thereafter. In hand with our results, Nafady-Hego et al. [12] found that most of the bacterial and fungal infections occurred within the 1st month. Similarly, Kim et al. [5] reported that most of the bacterial and fungal infections occurred within one month after LDLT, whereas most viral infections occurred after one month.
The frequently observed bacterial organisms in the 1st month were Klepsilla (17.1%) and Staphylococcus aureus (17.1%), and fungal strains were Candida albicans (5.7%). The commonest viruses after the 1st month were cytomegalovirus (23.8%) and Epstein-Barr viruses (19%).
Of the pre-operative data, only PELD/MELD score showed significant positive correlation with the frequency of the 1st month infection attacks (P < 0.05). However, Pre-LTx infectious state, pre-LTx steroid therapy, receiving hepatitis B vaccine and presence of growth failure showed no statistical difference between infection and non-infection group in 1st month (P > 0.05). On the other hand, Garcia et al. [13] found that infection was more frequent in transplanted patient suffering growth failure and pre-LTx infections.
Older age and higher BMI of the donors were associated with the occurrence of infection in the recipients during the 1st month post-LTx (P < 0.05). Máthé et al. [14] reported that, higher donor age and BMI has a deleterious impact on early allograft function and patient mortality. Advanced age impairs the regenerative capacity of the liver. The risk ratio increases from 1.00 (donor age < 40 years), to 1.17 (donor age 40-49 years), to 1.32 (donor age 50-59 years), to 1.53 (donor age 60-69 years), to 1.65 (donor age > 70 years). For that, whenever multiple donors are available "which is not always the case" the selection priority should be for those with younger age and lower BMI.
Prolonged anhepatic phase was found to be a significant risk factor for the occurrence of infection (P < 0.05) in 1st month; while other surgical and intraoperative parameters were nearly comparable in patients with and those without infection. Kusne et al. [15] found that roux-en-y biliary anastomosis, prolonged cold ischemia time and prolonged operative time (> 12 hours) were associated with increased risk of post-operative infection. The lack of evident significance for such parameters in our study may be due to the small number of patients.
We found that the period of ICU stay encompasses 88.9% of infection attacks in the 1st month post-LTx and 77.4% of the infection attacks during the total period of post-operative hospitalization. This red flag points out how much attention must be paid for such critical post-LTx period. Bouchut et al. [16] reported that prolonged ICU stay > 19 days was associated with increased risk of infection.
The distinction whether the patient is suffering infection or rejection is sometimes challenging as both may have fever, malaise, and elevated transaminases and bilirubin levels [17]. Furthermore, although liver biopsy is conclusive, it carries the risk of invasive procedures and time consuming to interpret [18]. In our study, we found that the mean levels of AST, ALT, ALKP and GGT were significantly higher in patients with rejection than in those with infection (P < 0.05), while total bilirubin and FKL showed no significant difference between both conditions. The highest accuracy were for ALKP (84.4%) followed by the GGT (80.2%). Combining these parameters together has improved the accuracy up to 88.4% which may facilitate the rapid decision for management of the suspected attack.
From previous results we can recommend that; special attention must be paid to the proper sterilization of the operation theater and the ICU belongings, close follow up and the use of sensitive monitors of infections must be employed in post-LTx patients. The donor selection priority should be for those with younger age and lower BMI. The socio-economic status of the recipient should be put in mind, so detailed patient education against daily infection risks is mandatory. Consider work-related issues especially if the patient deals with animals and reduce the risk of respiratory infections by avoiding crowded places and exposure to tobacco smoke. Early introduction of anti-rejection therapy is advised once significant elevation of AST, ALT, ALKP and GGT is noticed, before unavoidable graft loss occurs even if FKL is high or normal.
Conclusion
Infection was found to be a significant cause of morbidity after pediatric LTx. The most incriminated risk factors for occurrence of infection were related to the higher donor age and BMI, surgical complications and longer ICU stay. Transaminases and biliary enzymes, but not FKL, were significant discriminators between infection and rejection, with higher levels in the latter.
Acknowledgement
We thank the patients and investigators who participated in the included study. We also thank our colleagues, Pediatric Hepatology Department, National Liver Institute, Menofia University.
Compliance with Ethical Standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
-
Leonis MA, Balistreri WF (2008) Evaluation and management of end-stage liver disease in children. Gastroenterology 134: 1741-1751.
-
Tamura S, Sugawara Y, Kukudo N, Makuuchi M (2008) Systematic grading of morbidity after living donation for liver transplantation. Gastroenterology 135: 1804.
-
Souza MV, Barth AL, Alvares-da-Silva MR, Machado AR (2007) Infections after liver transplantation in adults: data from a university hospital in southern Brazil (1996-2000). Arq Gastroenterol 44: 128-132.
-
Snydman DR (2001) Epidemiology of infections after solid-organ transplantation. Clin Infect Dis 33 Suppl 1: 5-8.
-
Kim JE, Oh SH, Kim KM, Choi BH, Kim DY, et al. (2010) Infections after living donor liver transplantation in children. J Korean Med Sci 25: 527-531.
-
Singh N (2000) The current management of infectious diseases in the liver transplant recipient. Clin Liver Dis 4: 657-673.
-
Losada I, Cuervas-Mons V, Millán I, Dámaso D (2002) [Early infection in liver transplant recipients: incidence, severity, risk factors and antibiotic sensitivity of bacterial isolates]. Enferm Infecc Microbiol Clin 20: 422-430.
-
Shepherd RW, Turmelle Y, Nadler M, Lowell JA, Narkewicz MR, et al. (2008) Risk factors for rejection and infection in pediatric liver transplantation. Am J Transplant 8: 396-403.
-
Freeman RB Jr, Wiesner RH, Roberts JP, McDiarmid S, Dykstra DM, et al. (2004) Improving liver allocation: MELD and PELD. Am J Transplant 4 Suppl 9: 114-131.
-
Arora RS (2011) Pediatric liver transplantation in India: The complete picture? J Indian Assoc Pediatr Surg 16: 120.
-
Engelmann G, Schmidt J, Oh J, Lenhartz H, Wenning D, et al. (2007) Indications for pediatric liver transplantation. Data from the Heidelberg pediatric liver transplantation program. Nephrol Dial Transplant Suppl 8: 23-28.
-
Nafady-Hego H, Elgendy H, Moghazy WE, Fukuda K, Uemoto S (2011) Pattern of bacterial and fungal infections in the first 3 months after pediatric living donor liver transplantation: an 11-year single-center experience. Liver Transpl 17: 976-984.
-
García S, Roque J, Ruza F, González M, Madero R, et al. (1998) Infection and associated risk factors in the immediate postoperative period of pediatric liver transplantation: a study of 176 transplants. Clin Transplant 12: 190-197.
-
Máthé Z, Paul A, Molmenti EP, Vernadakis S, Klein CG, et al. (2011) Liver transplantation with donors over the expected lifespan in the model for end-staged liver disease era: is Mother Nature punishing us? Liver Int 31: 1054-1061.
-
Kusne S, Dummer JS, Singh N, Iwatsuki S, Makowka L, et al. (1988) Infections after liver transplantation. An analysis of 101 consecutive cases. Medicine (Baltimore) 67: 132-143.
-
Bouchut JC, Stamm D, Boillot O, Lepape A, Floret D (2001) Postoperative infectious complications in paediatric liver transplantation: a study of 48 transplants. Paediatr Anaesth 11: 93-98.
-
Cabrera R, Ararat M, Soldevila-Pico C, Dixon L, Pan JJ, et al. (2009) Using an immune functional assay to differentiate acute cellular rejection from recurrent hepatitis C in liver transplant patients. Liver Transpl 15: 216-222.
-
Lang M, Neumann UP, Müller AR, Bechstein WO, Neuhaus R, et al. (1999) Complications of percutaneous liver biopsy in patients after liver transplantation. Z Gastroenterol 37: 205-208.





