Bisphenol A (BPA) is being considered by the European Union as a substance of very high concern, occurring worldwide due to its wide application in many plastic products, building materials, coatings and epoxy resins. The toxicity of BPA in groundwater invertebrates is insufficiently understood.
Both acute (24 h) and chronic (28 d) toxicity was compared in Daphnia magna and ecologically important crustaceans in surface water (Gammarus fossarum, Eucyclops serrulatus) and groundwater (Niphargopsis casparyi, Proasellus slavus). Survival and spontaneous locomotory activity was recorded in real-time in the Multispecies Freshwater Biomonitor (MFB) and the new Microimpedance Sensor System (MSS) for Meiofauna.
The benthic copepod E. serrulatus was the most sensitive test species, followed by the groundwater species N. casparyi and P. slavus with G. fossarum and D. magna being the least sensitive species in acute tests. In chronic exposures the most sensitive species was P. slavus, whereas the amphipods and D. magna showed overall similar sensitivity. G. fossarum showed slightly more sensitive behavioral responses compared to N. casparyi, while D. manga behavior was affected by an age-dependent increase, masking potential negative effects of BPA on locomotory activity. Feeding activity of G. fossarum was more sensitive than molting and reproduction of D. magna. G. fossarum can be recommended for toxicity evaluation of groundwater habitats, as no standard test procedures and toxicity data for groundwater species are available. The organisms might also be used as biomonitors for landfill leachates, wastewater treatment plants, sewage plants and paper recycling plants.
Bisphenol A, Gammarus fossarum, Niphargopsis casparyi, Proasellus slavus, Eucyclops serrulatus, Daphnia magna
In 2016, REACH classified BPA as substance of very high concern, due to its toxic effects on reproduction (svhc: substances of very high concern, category 1B), which is being implemented from 1 March 2018 in Europe [1]. Moreover, BPA has been placed on the watch list of the European Water Framework Directive as xeno-estrogen micropollutant (www.umweltbundesamt.de).
The synthetic phenol 2,2 Bis (4-hydroxylphenyl) -propane (Bisphenol-A, BPA) is being used as additive in the production of plastic materials (mostly polycarbonate), phenol and epoxy resins, polyesters and polyacrylates [2]. It can be found in many products of daily life, such as CDs, polycarbonate bottles, thermal receipts, coatings of food tins and drinking water pipes, resin based dental sealing and epoxy glues [3]. BPA is being produced in huge amounts worldwide (e.g. 410.000 t/y in Germany) and occurs ubiquitous, even in dust and human urine samples [4].
BPA is released into the environment through sewage treatment effluents, landfill leachates and natural degradation of polycarbonate plastics [5]. The negative effects of BPA in vertebrates are various and include disruptions to the reproductive, immune and central nervous systems [6] as well as inducing developmental disorders in the brain and carcinogenesis, whereby numerous signaling pathways can be affected [2]. Moreover, endocrine disruption [7] and impairment of the glucose metabolism have been reported in zebrafish Danio rerio larvae [8] as well as reduced fish body length, induced oxidative stress and altered gene expression of immune-related genes in zebrafish [9]. The replacement products such as Bisphenol F and Bisphenol S [10] and degradation products such as BPA-mono/dimethyl ethers are toxic, too [11].
Wastewater treatment plants have a varying capacity to reduce BPA by 61-98%, however, the concentrations of degradation/transformation products may rise [3]. In surface waters, BPA concentrations range between 0.01 - 2.4 µg/L, most of the small German streams showed values below 0.05 µg/L, whereas large rivers like the Danube and Main can reach up to 130 µg/L resp. 50 µg/L (www.lfu.bayern.de) [12]. BPA levels in landfill leachates, wastewater treatment plants, sewage plants and paper recycling plants can reach of up to 33.5 mg/L [13,14]. Drinking water in China contains up to 6.5 ng/L BPA [15].
BPA can accumulate in sediments to 6 - 30 µg/Kg [16]. BPA uptake in human beings results mostly from food/beverage and thermal receipts, but also from dust. BPA has been rated as an endocrine disruptor, affecting both sexual and thyroidal hormone pathways.
Since 2011 BPA has been forbidden as a precautionary measure in feeding bottles for infants in the EU [1]. However, Japan has banned BPA already 20 years ago in all materials related to human consumption. Canada banned BPA in 2008 [1] after the U.S. National Toxicology Program (NTP) expressed the first concerns [17]. The European Food Safety Authority (EFSA) reduced the tolerable daily intake (TDI) of BPA in 2015 down to 4 µg/Kg. In France, the use of BPA has been banned for all food/beverage packaging in 2015.
Consulting the ecotoxicology database of US-EPA [18], numerous toxicity studies of several BPA-compounds (esp. CAS 79447 and 80057) have been performed on the zebrafish Danio rerio and Daphnia magna as standard species in ecotoxicology. A rough overview of the US ecotoxicology database shows that BPA does not seem to be highly acutely toxic on survival, growth and behavior of aquatic crustaceans with gammarids showing similar sensitivity as daphnids. However, no studies on groundwater crustaceans have been reported so far.
Groundwater habitats are characterized by lack of light, space and current, constant temperature and usually low pollutant levels. Groundwater invertebrates are mostly small, they show slow growth and long lifespan, thin cuticula, loss of eyes and pigments, slow metabolism and ability to starve long periods of food shortage [19-21]. Therefore, they are expected to react differently to xenobiotics compared to their epigean relatives. Currently there is a scientific debate if the standard aquatic test species, primarily daphnids, also protect groundwater invertebrate species or if there is a need to find groundwater test species and develop specific toxicity test guidelines for a safe risk assessment to fulfill the European Groundwater Directive.
The aim of this study is to provide toxicity data on BPA on groundwater crustaceans in comparison to surface water species and standard test species in aquatic ecotoxicology.
Both surface water and groundwater crustaceans were used and compared for the evaluation of BPA-toxicity in aquatic environments.
Gammarus fossarum (Crustacea, Amphipoda) represents a key ecological stream invertebrate in the whole Northern hemisphere due to its; (1) Wide geographical distribution; (2) High abundances; (3) Central position in aquatic food web and importance as decomposer; (4) Important role as bio indicator for saprobic water quality class II according to the European Water Framework Directive and; (5) Increased use as test species in ecotoxicology [19]. Organisms for tests and laboratory culture were originally collected in a small mountainous creek in Kreuzlingen, Switzerland (47.63311 °N, 9.16553 °E) and bred since then in the laboratory.
Daphnia magna (Crustacea, Phyllopoda) represents a key ecological species in lake ecosystems and has been used as standard test species in ecotoxicology for more than four decades. The initial organisms for tests and laboratory culture have been received from the University of Constance. The animals were cultured and bred at 20 ℃, 16:8 h photoperiod in medium according to ISO 6341 prepared with water from Lake Constance with Scenedesmus longispina as food (3x/week).
Eucyclops serrulatus (Crustacea, Copepoda) is primarily regarded as epigean benthic freshwater inhabitant, which also lives in the interstitial and in genuine and organically enriched groundwater habitats [22,23]. Organisms for tests were taken from our laboratory culture.
Niphargopsis casparyi (Crustacea, Amphipoda) are hypogean relatives of the epigean gammaridae. Niphargids have not been used in ecotoxicological testing as laboratory culturing has so far been unsuccessful.
Proasellus slavus (Crustacea, Isopoda) represents a small un-pigmented asellid with a life expectance of up to 15 years [24]. As hypogean relative to surface water Asellus spp. it co-occurred with N. casparyi in the groundwater monitoring site in about 50 m depth in the Rhine valley near Neuenburg, Southwest Germany (47.81272 °N, 7.54740 °E).
The culture of D. magna was performed according to ISO 6341. 800 mL medium dissolved in pre-filtered (100 µm) water from Lake Constance were filled in 1 L glass beakers with 5 animals at 20 ℃ with a 16 h:8 h photoperiod and 5 mL algae suspension of Scenedesmus longispina (Helbig Lebendkulturen) culture 3x/week, when the test medium was renewed. The culture was checked weekly for quality by determination of the sex of the animals under the microscope (Motic B3 Professional Series, 4 × 10 × 40).
The culture of G. fossarum was performed in a 20 L glass aquarium in a thermostat at both 10 ℃ (for comparison with the tests of the groundwater species) and at 20 ℃ (for comparison with D. magna), containing water from Lake Constance, pre-conditioned alder leaves and pebbles as substrate. The culture was kept and bred in the dark under constant aeration (oxygen saturation 95%). The water was renewed on a weekly basis and animals then fed with chironomid larvae (Poseidon-Aquaculture). The stygal crustaceans were collected in the field and maintained in Polyethylene boxes (40 × 30 × 20 cm) covered with parafilm at 10 ℃ in the dark with a few drops of fine detritus and in water from the groundwater sampling site for at least 4 weeks. Evaporating water was replaced on a weekly basis by water from Lake Constance.
Acute (24 h, 96 h) and chronic (16 - 28 d) toxicity tests were performed with the abovementioned crustaceans in a thermostat (at 10° and additionally 18° (N. casparyi, P. slavus, G. fossarum) without illumination, and for D. magna: 20 ℃ with 16:8 h photoperiod). For each species five organisms were placed in a beaker (250 mL) filled with 200 mL test water (Lake Constance water, drinking quality: pH 7.0, NH4: 0.05 mg/L, hardness 1.24 mmol, NO2: < 0.02 mg/L, NO3: 0 mg/L, PO4: 0.25 mg/L) and different BPA concentrations were added. The tests were replicated 3 - 4 times, the animals not fed during the acute tests. All experiments were accompanied by controls (Lake Constance water) and controls with ethanol, functioning as solvent (acute tests: 0.5 ml/L, chronic tests: 0.05 ml/L). The amount leaf eaten by the organisms (amphipods, isopods) was calculated as % leaf loss related to the number of the survivors in each beaker each week.
As the feeding habits of the stygal species are still unknown additionally 1 drop of fine detritus from the groundwater sampling site was added to each beaker. As groundwater organisms live in interstitial habitats 5 g fine pebbles (mixed sizes 1 - 3 mm, after previous incineration for 1 h, 500 ℃) were added as substrate and hiding places. G. fossarum demands higher oxygen saturation than groundwater species, therefore gammarid beakers were continuously aerated using standard aquarium pumps (ACO, Green Sun) with fine pipette tips gently dipped into the beakers to achieve 90 - 100% oxygen saturation.
During chronic toxicity tests with D. magna molting and reproduction was monitored daily according to the OECD guideline no. 211.
As BPA is poorly soluble in water, a stock solution was prepared with 50 mg/L of granulated BPA, dissolved in 1 mL Ethanol (96%) and 999 mL lake water. Then, the solution was placed inside a water bath (40 ℃) and stirred for one hour until the BPA was completely dissolved and the ethanol evaporated. For D. magna tests Daphnia medium was used instead of lake water. BPA solutions were prepared and changed every week to ensure a stable concentration during the experiments.
Each concentration level of BPA was tested in 3 - 4 replicates, depending on the amount of available test organisms: the field collected groundwater species were the bottleneck in the study. Beakers were covered with parafilm to minimize evaporation and hence change BPA concentration. Survival and behavior were monitored twice a week. Behavior was quantitatively recorded in lake water in eight randomly chosen organisms from each BPA concentration level, at selected exposure time intervals (acute tests: recorded continuously for 24 h in the test solutions; chronic tests: recorded for 2 h twice a week in lake water/medium) in the MFB (Multispecies Freshwater Biomonitor©) [25,26] or in the MSS (Microimpedance Sensor System© for E. serrulatus. The MSS is a new recording unit for small organisms of sizes around 1 - 1.5 mm [27]. In the chronic exposures behavior was recorded twice a week for a period of 2 h in lake water, in order to avoid BPA-spilling and ease of handling. Table 1 gives an overview over the toxicity tests.
Table 1: Overview of the toxicity tests with Bisphenol A (BPA). View Table 1
According to the OECD test guidelines, additionally reproduction (number of neonates per female) and molting was recorded daily in the chronic tests with D. magna.
BPA (CAS 1980-05-07, 97%, Alfa Aesar) levels were exemplarily verified in a wide range of concentration levels in; (1) The weekly prepared stock solutions and; (2) Directly after dosing to the experimental beakers and; (3) One week after exposure, i.e. before the weekly change of test solutions in the beakers to prove that (1) Solubility in Ethanol was sufficient to solve the BPA; (2) The experimental dilutions were correctly prepared and; (3) The loss of BPA during one week was negotiable. Stock solutions (50 mg/L) showed high recovery after 1 week (49 mg/L ± 1 mg/L). The loss of BPA in the experimental beakers after 1 week reached between 5 and 20% esp. at higher levels concentration levels.
Survival data from the acute exposures were estimated by a linear regression of log(x) and probit (y) transformed data in SigmaPlot. The EC50 for locomotory activity were estimated when possible with SigmaPlot with a 4-parameter logistic curve.
Behavior data were analyzed by non-parametric repeated measures ANOVA on ranks (Friedmann test) for time-dependent data or Kruskall-Wallis ANOVA to compare different groups for not time-dependent data (e.g. reproduction, molting of D. magna), followed by post-hoc pair wise comparisons (Tukey, Dunn's test).
G. fossarum is a highly mobile species and thus shows the highest activity, followed by the planktonic D. magna. The groundwater species, N. casparyi and esp. P. slavus showed lower locomotory activity as they live in the interstitial with limited space (Figure 1). The same holds for E. serrulatus, living in fine particulate sediment zones [27]. D. magna showed the highest variation in activity during the experiments. Generally, normal activity levels varied up to 15% over time in the controls. A significant BPA-dependent decrease in activity was therefore pre-defined as > 20% permanent difference in activity levels.
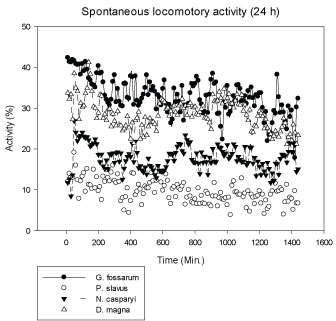 Figure 1: Mean spontaneous locomotory activity of different test species during 24 h real-time recording in the MFB (N: 8, means). X-axis: Time (Min.) of the whole recording period of 24 h. Y-axis: Percentage of locomotory activity spent by an organism during a recording interval (4 Min.). View Figure 1
Figure 1: Mean spontaneous locomotory activity of different test species during 24 h real-time recording in the MFB (N: 8, means). X-axis: Time (Min.) of the whole recording period of 24 h. Y-axis: Percentage of locomotory activity spent by an organism during a recording interval (4 Min.). View Figure 1
During the 28 d exposures D. magna grew fast to sexual maturity and their spontaneous locomotory activity significantly (p < 0.001, Kruskall Wallis test) increased with age (6 d: mean = 33.33 (SD = 6.33); 12 d: mean = 43.90 (SD = 2.5); 46 d: mean = 48.57 (SD = 3.64)). This was not the case in the slow growing surface and groundwater amphipods and isopod, which have much longer life cycles. Statistically, there is a significant difference (p < 0.05) among the organisms of different age: younger daphnids showed lower locomotory activity with a very high variability, whereas older organisms' activity increased and was more stable.
Stygal species are adapted to low and constant temperatures in groundwater habitats. In order to test whether the organisms also tolerated higher temperatures, which would allow to perform all tests on all species at the same temperature levels survival and activity of stygal species was tested at both 10 ℃ and 18 ℃. The activity of the stygal animals generally increased at higher temperature, however for P. slavus, it fell back to low levels after 2 weeks, followed by increased death (Figure 2). Therefore, it was decided to perform the toxicity tests with the stygal species and gammarids at 10 ℃, to avoid artifacts of unrealistic conditions and for comparisons, whereas the tests with daphnids were performed at 20 ℃ according to standard test procedure, and compared with tests with G. fossarum at 18 ℃ (which is the temperature of the long-term culture in the laboratory).
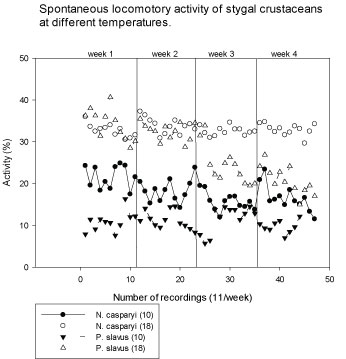 Figure 2: Temperature-dependent (10 versus 18 ℃) normal baseline activity of the stygal crustaceans N. casparyi and P. slavus (Means of 10-18 animals). Y-axis: Percentage of locomotory activity spent by organisms during a recording interval (4 Min.). X-axis: Number of subsequent recordings (11) once a week, over a period of 4 weeks. View Figure 2
Figure 2: Temperature-dependent (10 versus 18 ℃) normal baseline activity of the stygal crustaceans N. casparyi and P. slavus (Means of 10-18 animals). Y-axis: Percentage of locomotory activity spent by organisms during a recording interval (4 Min.). X-axis: Number of subsequent recordings (11) once a week, over a period of 4 weeks. View Figure 2
After 24 h, the small-sized copepod E. serrulatus was the most sensitive species, followed by the groundwater isopod P. slavus, then G. fossarum, before D. magna and the stygal N. casparyi (Table 2). A more detailed comparison between the median lethal concentrations, LC50s (at 18 - 20 ℃) for several days was calculated from a separate experiment for the two standard species D. magna and G. fossarum, showing both species' similar sensitivity to BPA after 24 h (G. fossarum: 11.86 (8.6 - 16.2) mg/L; D. magna: 11.9 (9.8 - 14.8) mg/L, however, increasing sensitivity of G. fossarum after already 48 h (G. fossarum: 6.2 (4.6 - 8.4) mg/L; D. magna: 8.9 (6.9 - 11.5) mg/L, and even without overlap of the confidence intervals (95% CI) after 96 h (G. fossarum: 3.4 (2.3 - 5.2) mg/L; D. magna: 8.2 (6.2 - 10.9) mg/L. Moreover, the LC50 24 h values for both species were comparable to those of the 1st test at 10 ℃.
Table 2: LC50 values (R2), EC50 ( ± SE) and ET20 values generated from the acute toxicity tests. View Table 2
Under BPA exposure the activity of most species declined with increasing concentration levels and decreasing response time of the decline. The median effect concentrations, EC5024 h values (i.e. the concentration with a 50% decrease in activity) and the ET20 (i.e. the time of 20% decrease in activity at the lowest effective concentration) are found in (Table 2). P. slavus was the most sensitive species regarding mortality within 24 h and rapidly decreasing activity already after 6 h in 2.5 mg/L BPA. However, regarding the EC50 values after 24 h, N. casparyi was the most sensitive species with 5 mg/L BPA being the threshold for 20% decrease in activity, compared to 10.3 mg/L in D. magna, 13.5 mg/L in G. fossarum and 25 mg/L BPA in P. slavus.
The chronic exposures revealed P. slavus to be the most sensitive species regarding survival and behavior effects of BPA (Table 3). Moreover, there was a trend of D. magna to be slightly more sensitive than the amphipods G. fossarum and N. casparyi regarding mortality. However, behavior of D. magna could not be evaluated as there was an overall increase in activity with age in all concentration levels and the controls masking potential effects of BPA.
Table 3: Comparison of chronic effects (28 d) of BPA on different crustaceans. View Table 3
G. fossarum consumed alder leaves and there was a significant decrease in feeding activity in all BPA-exposures and with increasing BPA concentration (Kruskall Wallis test p < 0.01; Figure 3). A 50% decline in feeding activity was almost reached at 0.5 mg/L BPA.
 Figure 3: Feeding activity of Gammarus fossarum, exposed to BPA (28 d). Y-axis: Leaf loss (cm2). Means (R = 3, N = 5) and SD bars. X-axis: BPA concentrations (mg/L). View Figure 3
Figure 3: Feeding activity of Gammarus fossarum, exposed to BPA (28 d). Y-axis: Leaf loss (cm2). Means (R = 3, N = 5) and SD bars. X-axis: BPA concentrations (mg/L). View Figure 3
Daphnia magna showed no effects in molting frequency under BPA exposure (0.03, 0.3 and 3 mg/L) (Kruskal Wallis, not significant). However, effects of BPA on reproduction in D. magna could be seen at the end of the 28 d exposure at ≥ 3 mg/L BPA, even though not significant (Kruskall Wallis, not significant). The cumulative number of neonates was variable within the replicates of one treatment, the highest number of neonates was reached at 0.03 mg BPA/L, the lowest in the control (Table 4).
Table 4: Cumulative number of neonates per female in D. magna exposed to different BPA concentrations (mg/L) over 28 d. Means (R = 3, N = 10) and SD (standard deviation). View Table 4
BPA can be found in many polycarbonate plastics and epoxy resins [28-30]. BPA has been reported to be acutely toxic and function as an endocrine disruptor in aquatic organisms [28,31].
In the acute toxicity tests (24 h) the copepod E. serrulatus was the most sensitive test species regarding survival and rapid behavioral responses. This might be due to its small size, its rapid generation cycle and its feeding habits as fine particle feeder. However, D. magna larvae of similar size as E. serrulatus were less sensitive. They feed on planktonic algae and have slower growth and a longer life cycle than the copepod, hence they might take up BPA less rapidly during short exposure times. Regarding the larger crustacean species, the groundwater species P. slavus reacted very rapidly and sensitively to BPA, compared to N. casparyi and G. fossarum. Regarding the overall effect on locomotory behavior, the EC50-24 h, N. casparyi was the most sensitive species. These results show, that groundwater crustaceans tend to react both faster and more sensitive to BPA short-term exposure than the surface water representative G. fossarum. Generally, groundwater species have a thinner and transparent cuticula, i.e. less protection from the surrounding aquatic environment.
The surface water crustaceans G. fossarum and D. magna showed similar sensitivity in the more detailed and direct comparative acute toxicity test (24 - 96 h) at 18 - 20 ℃. Whereas the 24 h LC50 for D. magna (1 - 2 mm) was 11.9 mg/L (9.58 - 14.8), the value for G. fossarum (5 - 7 mm) was 13.41 mg/L (9.7 - 18.43). In the literature, LC50 values for 24 h exposures were reported in D. magna neonates as follows: Brennan, et al. [32]: 8.57 mg/L (8.28 - 8.86), Plahuta, et al. [33]: 12.5 mg/L (11.3 - 14.1,) Jemec, et al. [34]: 21 mg/L (20.8 - 23.8) for organisms of similar sizes. Moreover, acute toxicity (LC50 24 h) in neonates of D. magna required 7.9 mg/l, [35]. Watts, et al. [36] reported a 24 h LC50 of 12.8 mg/L in G. pulex. The values of this study supported literature data.
After 24 h of acute exposure, G. fossarum became more sensitive to BPA than D. magna, as the 48 h LC50 of G. fossarum decreased to 6.67 mg/L (4.8 - 9.1) compared to 8.94 mg/L (6.9 - 11.52) in D. magna. Similar results were previously found for G. pulex (5.6 mg/L, Watts, et al. [36]), and D. magna (7.57 mg/L, Brennan, et al. [32]). The few literature studies on freshwater amphipods G. pulex and G. fossarum revealed effects on survival at 12.8 mg/l (LC50 24 h), 4 mg/l (LC50 72 h), 1.9 mg/l (LC50 96 h) and 1.4 mg/l (LC50 10 d) [36].
The difference in sensitivity between G. fossarum and D. magna increased from 48 h to 96 h exposure even further. This shows that test duration, even in acute tests, can drastically affect the test results. Up to now, D. magna was always regarded the most sensitive aquatic sentinel species in aquatic ecotoxicology. Moreover, it was always believed that small organisms should be more sensitive than larger organisms due to the larger surface/volume ratio. However, in this study the small daphnids were less sensitive than the larger gammarids towards BPA. Moreover, filter feeders such as daphnids should be more sensitive than detritivores, as they filter great amounts of water (and dissolved toxins) through their body. The moderate toxicity of BPA to D. magna (EC50-48 h: 10 mg/L) was also stated by Chen, et al. [37].
The marine rotifer Brachionuskoreanus showed effects of BPA, BPF and BPS, such as increased intracellular ROS (reactive oxygen species) and GST activities and gene expression modulation of cytochrome P450 [10]. Similar biochemical responses might be underlaying the observed reduced mortality and locomotory activity in the crustacean species of the current study.
Moreover, fish toxicity studies on BPA revealed an LC50 (72 h) for Poecilla reticulata of 1.6 mg/L, which is more sensitive than the previously reported toxic range for fish of 6 - 17.9 mg/L [38]. The acute effects (48 h) of BPF in zebrafish larvae Danio rerio included disrupted glucose metabolism at > 10 µg/L, showing the rapidity of physiological responses at low concentration levels [8].
However, the acute toxicity levels found in this study are higher than the BPA levels reported in the field. The highest BPA concentrations in surface waters were 21 µg/L [39] and 17.2 mg/L in landfill leachates [40]. BPA levels in surface water in Germany range from 0.5 - 5 µg/l, in a stream in an industrial area of Norway a concentration of 43 µg/l was reported, whereas values in water of Lake Constance taken for drinking water were as low as 2 ng/l [3].
The sensitivity of the studied surface and stygal crustacean species might therefore help to indicate landfill leachate spills or water quality in areas close to landfills or water treatment plants. On the other hand, the copepod E. serrulatus might be used to monitor BPA pollution in surface water, e.g. taken for drinking water preparation.
During chronic BPA exposures the stygal isopod Proasellus slavus was the most sensitive species regarding both survival and behavior, followed by G. fossarum (esp. feeding behavior), and last Niphargopsis casparyi. Regarding survival, G. fossarum was as sensitive as D. magna. (G. fossarum LC50 28 d: 0.80 ± 0.12 mg/L; D. magna 0.74 ± 0.25 mg/L). Watts, et al. [36] found an LC50 10 d for G. pulex at 1.49 mg/BPA/L. Chronic exposures (21 d) revealed an increased number of daily molting in D. magna at 0.02 mg/L, increased dry weight at 2 mg/L, effects on body length at 8.2 mg/L and fecundity at 7.3 mg/L [35].
The No Observed Effect Concentration (NOEC) for stimulation of substance avoidance was found in G. pulex at 8.4 mg/L BPA, the Lowest Observed Effect Concentration (LOEC) being 19.4 mg/L (CAS 80057). G. fossarum responded sensitively with decreased feeding activity as alder leaf loss of about 40% was found in all BPA exposures, however, significant differences were only found at ≥ 1 mg/L. In BPA concentrations of 2 and 3 mg/L, the reduction of feeding activity was 75% and 95%, respectively, indicating high impact of BPA compared to the control (< 0.05). The feeding behavior of invertebrates is a very sensitive parameter. The reduction of the feeding activity is among the first responses to stress or environmental pollution [41].
N. casparyi and P. slavus starve for long periods as an adaptation to stygal environmental conditions. As feeding habits are still not known, both fine detritus from the sampling site and alder leaves were added, however the leaves were not touched by these species.
In arthropods, molting is necessary to allow for growth, being regulated by ecdysteroid hormones [42]. In this study, the cumulative molting of organisms was counted as D. magna has a short life cycle with many moltings. This was not possible in the tests with the amphipods and the isopod, as they have longer molting intervals, generating too little data for statistical analysis.
D. magna showed ca. 9 molts per female over 28 d exposure, supporting findings by Brennan, et al. [32], where on average females molted 9 times in a 21- day exposure to BPA. However, no significant differences between the molting of organisms exposed to BPA and the control could be seen. Mu, et al. [42] reported inhibition of molting in D. magna in BPA concentrations ranging from 5 to 10 mg/L. This is consistent with the results found in this study, as the molting of Daphnia magna exposed to concentrations of 3 mg/L and lower was not affected. However, Li, et al. [35] reported molting in D. magna to be inhibited at already 0.02 mg/L.
Another important parameter in the standard test procedures of D. magna represents the reproduction, which might be affected by BPA, known as endocrine disruptor for fish and amphibians and esp. snails [43]. D. magna has a short life cycle and reproduces several times during the 28 d exposure. The cumulative number of neonates per female was lowest in the control and higher in all other treatments, indicating rather an effect of ethanol than BPA. Ethanol might reduce bacterial and fungal growth in the beakers, hence support growth and reproduction of daphnids. As the number of neonates was similar in all BPA concentrations, no dose-dependent adverse effects of BPA on the organisms were observed. The results agree with those by Brennan, et al. [32] who counted around 70 neonates per female on a 21 d exposure to BPA. Jemec, et al. [34] found BPA affected the reproduction of D. magna only above 3.45 mg/L. Li, et al. [35] reported for D. magna under chronic exposure to BPA, lignin-derived (LD)-BPA and mixed exposures drastic adverse effects of the mixture at 2 mg/L on fecundity and enzyme activities. Lee, et al. [44] found adverse effects on reproduction in the midge C. riparius as well as increased DNA damage at 1 mg/L BPA. The aquatic oligochaete Lumbriculus variegatus responded to chronic exposures to BPA or BPS with retarded regeneration ability after fragmentation and increased blood vessel pulse rates at > 10 - 6 M [45].
After 28 d of exposure the snail P. antipodarum showed an increased number of embryos in the brood pouch at 0.04 mg/L [43]. Whereas survival of the nematode C. elegans was not affected by BPA (up to 10 µM), head thrashes (as a measure for locomotion) was decreased at 0.001 µM BPA already during 10 d of exposure [46]. BPA impaired the reproduction of the oligochaete Eisenia fetida as number of juveniles decreased at 1 mg/L, whereas mortality increased at 2 mg/L (14 d) [47].
Endocrine disruptive effects have been reported for fish and amphibians at concentrations around 16 µg/L, whereas aquatic insects and crustaceans appear to be more tolerant with effect levels between 100 - 3146 µg/L [3].
Effects on reproduction were found in Japanese Medaka after 60 d of exposure at levels of 1.5 mg/L, such as a reduced number of broods and total number of eggs [48]. No effects of BPA levels up to 1 mg/L were found in Danio rerio survival (120 d) [9]. However, Wei, et al. [7] proved adverse effects of BPS on the thyroid endocrine system with altered T3 and T4 levels, delayed development, neurotoxicity and reduction in motility at levels below 0.1 mg/L BPS in a 120 d long study. Such multiple effects of BPS in zebrafish were also reported by Gu, et al. [49], including decreased locomotory behavior, enhanced apoptosis, altered renal structure and increased oxidative stress at > 0.3 mg/L.
This study provides for the 1st time comparative toxicity data (acute, chronic) of BPA on both surface water and groundwater crustaceans.
E. serrulatus proved to be the most sensitive species in acute exposures to BPA regarding survival and locomotory behavior and might be used to monitor acute toxic pulses of bisphenols.
The most sensitive macro-crustacean species was the groundwater isopod P. slavus, in both acute and chronic exposures. P. slavus might be used for long-term monitoring of chronic low-dose exposures, e.g. in groundwater and drinking water.
G. fossarum (5 - 7 mm) proved to be more sensitive to BPA compared to the standard test species D. magna (1 - 2 mm) regarding survival, behavior and esp. feeding as the most sensitive parameter.
Skillful support in the performance of the acute and chronic toxicity tests is acknowledged to J. Mahlke (Eucyclops serrulatus), J. Ritzel, M. Urban and C.O.M. O'Dogherty (Gammarus fossarum, Niphargopsis casparyi, Proasellus slavus, Daphnia magna). Dr. M. Sengl (LfU, Augsburg, Bayern) and A. Thoma and A.L. Schneider (TZW Karlsruhe) kindly performed the chemical analysis of the water samples from the experimental setup. The staff at the Limnological Insititute, University of Constance, Prof. K.O. Rothhaupt, kindly provided starter cultures of D. magna.
The project was co-funded by the German Federal Ministry of Education and Research (BMBF) as part of the funding measure "Regional Water Resources Management for Sustainable Protection of Waters in Germany" - ReWaM (GroundCare: funding code 033W037H). The funding body did neither influence study design, performance, interpretation nor article writing. The copyright of the data is owned by LimCo International GmbH.