The thoracolumbar region (T11 to L2) is more susceptible to injury than other parts of the spine. Approximately 50% of all vertebral body fractures and 40% of all spinal cord injuries occur from T11 to L2 [1,2]. Although basic principles of diagnosis and treatment are established, there are controversies concerning diagnosis and treatment, including classification, indications for surgery and approach, as well as long-or short-segment of fixation and fusion [3-7].
Posterior pedicle screw-based instrumentation and fusion is a widely accepted procedure to restore alignment and achieve instant and long term segmental stability of the injured spine through fusion, which leads to permanent loss of motion in the fused segment [8-11]. Balance should be achieved between keeping the range of motion (ROM) of the spine and avoiding the failure of instrumentation resulted from insufficient fusion [12], but principles focusing on the level of fixation and fusion are in need. To optimize fusion strategy, the key factors determine the outcome of surgery should be studied carefully. Since the integrity of the vertebra and PLC is of importance for maintaining the supporting function of the spine and neural function determines the requirement of mobility, we hypothesize that the fixation and fusion strategy should be made based on the severity of vertebral endplate and PLC injury as well as spared neural function. Here we report a prospective study to support this hypothesis. From January, 2004 through December, 2013, 204 patients with thoracolumbar fracture were treated with posterior pedicle screw instrumentation but different fixation and fusion level strategy based on the injury severity of the vertebral endplate and the integrity of the PLC. In this prospective study, we found that the vertebral endplate, PLC and neural function play key roles in fusion strategy and fusion should be limited to the segment with severe injury of the vertebral endplates or ruptured PLC.
ROM: Range of Motion; L2: Lumbar Vertebra 2; T11: Thoracic Vertebra 11; PLC: Posterior Ligamentous Complex; ASIA: American Spinal Injury Association; ADL: Activities of Daily Living; MRI: Magnetic Resonance Imaging; FSUs: Functional Spinal Units; TLSO: Thoracolumbosacral Orthosis; DVT: Deep Vein Thrombosis; AVBH: Anterior Vertebral Body Height
The study was designed and conducted by two senior surgeons with approval from the ethics committee of the Third Military Medical University in December, 2003 and all the informed consents to participate in the study have been obtained from participants. From January 2004 through December 2013, 204 patients (23-52 years old, average is 37.8) with traumatic thoracolumbar injury treated by the two senior surgeons (Figure 1) were enrolled. The inclusion criteria were high energy injury and only posterior surgery is needed. Exclusion criteria consist of pathological or osteoporotic vertebral fracture due to low energy trauma; a history of previous surgery at the site of injury. Neurological deficit, major fractures at other sites and substantial associated injuries requiring priority treatment were not criteria for exclusion.
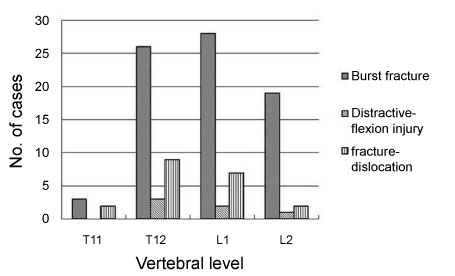 Figure 1: The types and distribution of thoracolumbar fractures. The types of fractures were analyzed according to Denis' classification of fractures and the vertebrae involved were recorded. View Figure 1
Figure 1: The types and distribution of thoracolumbar fractures. The types of fractures were analyzed according to Denis' classification of fractures and the vertebrae involved were recorded. View Figure 1
The indications for surgical treatment included distractive-flexion injuries; fracture-dislocations and burst fractures with > 20° local kyphosis and/or > 50% vertebral body collapse. The patients were informed all details about whether fusion was to be performed, which segments were to be fused, possible changes of the strategy during surgery, and implant removal in case non-fusion and selective fusion.
To fuse or not: Major factors for fusion strategy making included the integrity of the vertebral endplates involved; the preoperative integrity of the posterior ligamentous complex (PLC) revealed with MRI and whether this would be affected by decompression; preoperative neurological status ranked by the American Spinal Injury Association (ASIA) Standard Neurological Classification of Spinal Cord Injury and the feasibility to return to activities of daily living (ADL). In the present study, patients with intact or moderately injured endplates but intact PLC were subjected to posterior surgery without fusion, termed as "non-fusion" procedure (Figure 2), for those with obviously displaced fractures of the endplate and/or ruptures of the PLC, fusion was employed with instrumentation (Figures 3 and Figure 4). The integrity of the PLC was evaluated by physical examination, preoperative magnetic resonance imaging (MRI), and verified intraoperatively.
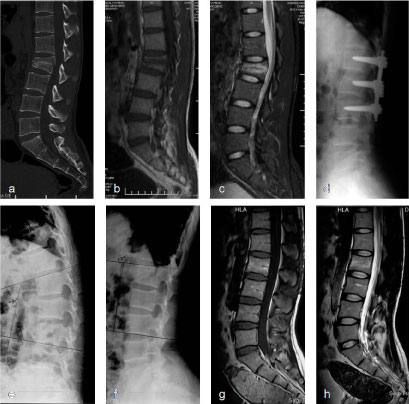 Figure 2: Typical radiograph of representative non-fusion cases. Male, 29-years-old, L2 burst fracture and bilateral calcaneal fractures without neural dysfunction resulted from a 3-meter-highfalling. ORIF of the L2 fracture without fusion via a posterior approach was conducted on the day of injury and ORIF of the calcaneal fractures on the 10th day. Normal ROM of the spine regained after instrument removal at 9 months and the patient returned to work without limitation and pain at last follow-up 37 months after first surgery. a) Preoperative sagittal computed tomography (CT) reconstruction shows the fracture line crossing the mid-portion of the vertebral body. Local kyphosis was 21° and loss of anterior vertebral body height (AVBH) was 54%; b,c) Magnetic resonance imaging (MRI) showed that both endplates of L2 were intact (b: T1 weighed image; c: T2 weighed image); d) The local curve (kyphosis of 6°) and vertebral body height (95% of AVBH) was restored 8 months after surgery; e,f) Lateral flexion-extention radiograph (e: flexion; f: extention) showed the ROM of L1-L3 was 32° 15 months after first surgery; g,h) MRI showed normal signal and shape of the L1/2 and L2/3 discs at 15 months after first surgery [19]. View Figure 2
Figure 2: Typical radiograph of representative non-fusion cases. Male, 29-years-old, L2 burst fracture and bilateral calcaneal fractures without neural dysfunction resulted from a 3-meter-highfalling. ORIF of the L2 fracture without fusion via a posterior approach was conducted on the day of injury and ORIF of the calcaneal fractures on the 10th day. Normal ROM of the spine regained after instrument removal at 9 months and the patient returned to work without limitation and pain at last follow-up 37 months after first surgery. a) Preoperative sagittal computed tomography (CT) reconstruction shows the fracture line crossing the mid-portion of the vertebral body. Local kyphosis was 21° and loss of anterior vertebral body height (AVBH) was 54%; b,c) Magnetic resonance imaging (MRI) showed that both endplates of L2 were intact (b: T1 weighed image; c: T2 weighed image); d) The local curve (kyphosis of 6°) and vertebral body height (95% of AVBH) was restored 8 months after surgery; e,f) Lateral flexion-extention radiograph (e: flexion; f: extention) showed the ROM of L1-L3 was 32° 15 months after first surgery; g,h) MRI showed normal signal and shape of the L1/2 and L2/3 discs at 15 months after first surgery [19]. View Figure 2
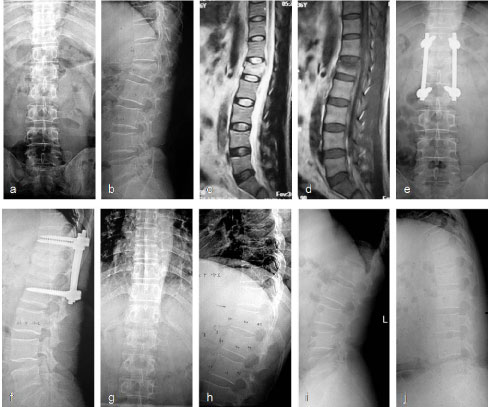 Figure 3: Typical radiograph of representative selective fusion cases. Male, 49-years-old, L1 burst fracture without normal neural function caused by a stair slipping. T12-L2 were instrumented and T12-L1 were performed selectively posterolateral fusion. Implants were removed 12 months later. a-d) Preoperative radiographs show a L1 burst fracture, local kyphosis of 22° and anterior vertebral body height (AVBH) loss of 47% (a,b); c,d) MRI shows the ruptured upper endplate and intact lower end plate; e,f) The spinal column was realigned with T12-L2 instrumentation and T12-L1 fusion; g,h) Instrument was removed 12 months later and fusion of T12-L1 was confirmed during surgery; i,j) Extension neutral flexion radiographs 13 months after implant removal show fusion of the T12-L1 segment. ROM in the L1/2 segment was 6° [19]. View Figure 3
Figure 3: Typical radiograph of representative selective fusion cases. Male, 49-years-old, L1 burst fracture without normal neural function caused by a stair slipping. T12-L2 were instrumented and T12-L1 were performed selectively posterolateral fusion. Implants were removed 12 months later. a-d) Preoperative radiographs show a L1 burst fracture, local kyphosis of 22° and anterior vertebral body height (AVBH) loss of 47% (a,b); c,d) MRI shows the ruptured upper endplate and intact lower end plate; e,f) The spinal column was realigned with T12-L2 instrumentation and T12-L1 fusion; g,h) Instrument was removed 12 months later and fusion of T12-L1 was confirmed during surgery; i,j) Extension neutral flexion radiographs 13 months after implant removal show fusion of the T12-L1 segment. ROM in the L1/2 segment was 6° [19]. View Figure 3
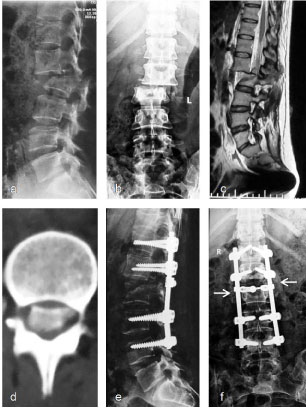 Figure 4: Typical radiograph of representative whole fusion cases. Female, 46-years-old, suffered multiple injuries in a road traffic accident. Dislocation occurred at the L1/2 level, with simultaneous burst fracture of L2 and complete loss of neurological function below the L1 level. Whole fusion surgery with long-segmental instrumentation and posterior decompression was undertaken. The dural sac was totally lacerated at the level of the dislocation and the cauda equina was disrupted. The function of the L1 and L2 roots had recovered partially and the supporting ability was normal in daily activities without back pain 3 months after surgery. a-c) Preoperative radiographs show dislocation of the L1 and discontinuity of the spinal cord at the L1/2 level in both the sagittal (a), coronal (b) plane and MRI (c); d) CT shows dislocated bone fragment in the spinal cannel; e,f) Postoperative radiographs at 16 months show maintained realignment at the L1/2 level (e). Fusion was achieved between the L1/2 transverse processes (arrow) and the T12–L1/L2–L4 laminae (f). Local kyphosis was 15°. Loss of anterior vertebral body height was 45% [19]. View Figure 4
Figure 4: Typical radiograph of representative whole fusion cases. Female, 46-years-old, suffered multiple injuries in a road traffic accident. Dislocation occurred at the L1/2 level, with simultaneous burst fracture of L2 and complete loss of neurological function below the L1 level. Whole fusion surgery with long-segmental instrumentation and posterior decompression was undertaken. The dural sac was totally lacerated at the level of the dislocation and the cauda equina was disrupted. The function of the L1 and L2 roots had recovered partially and the supporting ability was normal in daily activities without back pain 3 months after surgery. a-c) Preoperative radiographs show dislocation of the L1 and discontinuity of the spinal cord at the L1/2 level in both the sagittal (a), coronal (b) plane and MRI (c); d) CT shows dislocated bone fragment in the spinal cannel; e,f) Postoperative radiographs at 16 months show maintained realignment at the L1/2 level (e). Fusion was achieved between the L1/2 transverse processes (arrow) and the T12–L1/L2–L4 laminae (f). Local kyphosis was 15°. Loss of anterior vertebral body height was 45% [19]. View Figure 4
To fuse fully or selectively: For patients who might be able to return to normal activity (neural function ranked from Frankel C and better), fusion was confined to segments or functional spinal units (FSUs) with severely damaged endplates and/or PLC, with short-or long-segment fixation. This strategy was termed as "selective fusion" (Figure 3). For patients without sufficient neural function for independent walking, fusion were undertaken in all fixed segments, termed as "whole fusion" (Figure 4).
All instrumentation and fusion were undertaken through posterior approach. All facet joint capsules of non-fusion levels were kept intact during exposure. Pedicle screws were inserted. The levels to be fixed were determined according to the type and severity of the fracture and the patient's weight. Laminectomy was performed when decompression of neurological elements was necessary. The facets, laminae and pars interarticularis at fusion levels were decorticated and grafted with autogenous bone. Incision was closed with drainage.
Patients were advised to rest in bed for 2 days to 2 weeks following free ADL with a thoracolumbosacral orthosis (TLSO). During bed rest, physical treatment (active movement of the limbs and passive massage of muscles) was given to prevent the development of deep vein thrombus (DVT). No anticoagulation drugs were administered.
In non-fusion or selective fusion cases, the implants were removed as soon as bony union or fusion was confirmed by X-ray examination. Retention of implants was recommended for whole fusion cases but decided by patients.
All patients were evaluated at 1 week and 3, 6 and 12 months after surgery, and followed up annually thereafter. The minimal duration of follow-up was 24 months.
Clinical evaluation: Complications related to surgery were recorded. A back pain scale [13], the supporting ability of the involved segments and back stiffness (Table 1) were evaluated at the last follow-up visit. Three of the thirteen factors comprising the Low Back Outcome Score [14]-pain scale, resting and painkiller usage-were chosen for the clinical evaluation in our study. We considered the other factors to be significantly affected by neurological function.
Table 1: Back pain scale and supporting ability of the back. View Table 1
Radiological evaluation: All patients were followed up with plain X-rays at 1 week, 3, 6, 12 months and annually thereafter. CT was taken at 6 and 12 months. MRI was taken to evaluate soft tissues as needed. Radiographic images were evaluated by the two senior surgeons. The angle of local kyphosis was measured on lateral plain radiographs or sagittal CT scans using the standard Cobb technique. Loss of anterior vertebral body height (AVBH) was calculated as a percentage. Loss of kyphosis correction and AVBH were recorded at the last follow-up visit [15]. Fusion or healing of vertebral fractures was determined by CT scan at 6 and 12 months after surgery. Fusion was also corroborated during implant removal surgery in the selective fusion and whole fusion groups. For patients who underwent non-fusion or selective fusion procedures, segmental mobility was examined during implant removal surgery and by lateral flexion-extension radiographs at several months after implants removal.
Statistical differences among testing and control groups were analyzed by Student's t-test and one-way analysis of variance (ANOVA) followed by Bonferroni's multiple comparison post-test. The level of statistical significance was set at P < 0.05. All analyses were performed using GraphPad Prism v5.0 (GraphPad Software, Inc. USA).
One hundred and thirty-three consecutive patients with thoracolumbar fractures were enrolled in this study. Twenty-one patients followed up less than 6 months were excluded, comprising 8 cases of non-fusion, 18 selective fusions and 16 whole fusions. There was no difference in the age, sex among groups. The reason for loss to follow-up was change of contact information due to the rapid urbanization of mainland and frequent migration of populations. At the last follow-up, 204 cases (77%) were available at a mean of 31 months after surgery (range 24-53 months). The ratio of men to women was 4:1 (162 males and 24 females). Mean age at the time of injury was 38 years (range 18-58 years). Causes of trauma consist of falling from height (64%), traffic accidents (33%), firearm attack and hitting by falling objects (3%). The most affected vertebrae were T12 (37%) and L1 (36%) and the common types of fracture were burst fracture (75%), fracture-dislocation (20%) and distractive-flexion injury (5%) (Figure 1). The neural status before surgery and at last follow-up was ranked according to ASIA scale (Table 2). Results showed that most patients (79%) preserved motor function (Rank C, D or E) despite of severe injury to the bone, which testified the necessity of surgery to restore the spine support for spared neural function. Postoperative CT showed that bony healing was achieved in 6-9 months, while fusion was achieved within 1 year, usually at approximately 9 months. Asymptomatic malposition of three pedicle screws was detected in two patients and no revision surgery was required.
Table 2: Method of fusion employed according to type of fracture and preoperative neurological status. View Table 2
Fourteen non-fusion patients underwent surgery for implant removal within 1 year (8-11 months) since the first operation; the other four underwent removal surgery at 24 months and 35 months. One hundred and ten selective fusion patients and 30 whole fusion patients underwent implant removal within 3 years (range 15-32 months). Solid fusion was confirmed during implant removal surgery and was consistent with the CT scans.
At the last follow-up visit, 178 patients (87.25%) scored "excellent" on the back pain scale and for the back's supporting ability; 26 patients (12.75%) ranked "good" (Table 3).
Table 3: Outcome of back pain scale and supporting ability of back. View Table 3
Weakness and soreness at the incision site after implant removal surgery were common complaints, usually remitted spontaneously within weeks to months. 22 whole-fusion patients (22/76, 28.9%) complained of stiffness at all involved levels. 12 selective-fusion patients (12/110, 10.9%) complained of stiffness before implant removal and 4(4/110, 3.6%) had residual stiffness after removal surgery. Back stiffness was not reported in the non-fusion group (p < 0.01, compared with the former two groups), though obvious loss of segmental mobility was detected in the two patients who underwent late removal surgery. The result indicates that fusion results in stiffness in the involved levels, while keeping mobility alleviates the stiffness.
Preoperative, postoperative and last follow-up radiographs showed that mobility of the spine in non-fusion group obtained the best ROM (p < 0.01), while the non-fused segments preserved mobility in the selective fusion group who underwent prompt implant removal (within 1 year), but decreased when implant removal surgery was delayed over 1 year. The all cases in the whole fusion group lost all mobility in the segments. The correction of the anterior vertebral body height and kyphosis was achieved in all groups (Table 4), while the loss of kyphotic correction was more significant in the selective fusion group than other groups (p < 0.05, compared with the other two groups). Notably, loss of kyphosis correction occurred predominantly in the disc space rather than in vertebral height.
Table 4: Radiological parameters for each type of surgery. View Table 4
Fusion strategy for thoracolumbar fracture is inconsistent [13,16-21]. The present study shows that the integrity of the vertebral endplate and PLC and the spared neural function play major roles in setting fusion strategy. To fulfill the balance between gaining sufficient supporting of the spine and reserving mobility of FSUs, special attention should be paid to limit fusion to the segment of severely injured endplate and PLC in patients with neural function for ADL, which means non-fusion or selective fusion surgery are recommended for patients with intact endplates and PLC, but no severe neural function deficit. Fracture lines usually cross bony structures with intact endplates and PLC as well as normal neural function in thoracolumbar fracture patients, the goal of treatment is to align the spine column and restore the height of vertebral body, and fusion is contraindicated [16-18]. We suggest keeping facet joint capsules intact to guarantee the integrity of the functional tripod at non-fusion levels [14,15]. However, delayed implant removal leads to unintended arthrocleisis of the fixed segments, indicating that implant removal should be conducted once bone union is confirmed. Since the endplate plays an important role in the nutrition of intervertebral discs [22] and endplate injury is strongly associated with disc degeneration [23], we suggest fusing the FSU to eliminate motion-associated back pain when it bears load in case of patients with severely injured endplates. In our study, selective fusion surgery achieved expected clinical outcome and no medicine was needed for back pain. In some patients with split fractures of the vertebral body, we fused segments with a severely damaged endplate (usually the cephalic) and left the other (usually the caudal) alone. However, CT showed that both discs exhibited signs of the "vacuum phenomenon" 6-9 months later, which suggests accelerated disc degeneration and potential chronic back pain in years.
The PLC is an important factor for segmental stability and difficult to heal when injured [3]. We suggest fusing the involved FSU to avoid ligamentous instability, while others reported non-fusion strategy for all thoracolumbar fracture even with ruptured PLC [18]. We notice that the ROM measured with dynamic lateral radiographs in that paper is largely dependent on the intact level (caudal), and the level with distractive flexion injury is ankylosed attributed by spontaneous fusion. In present study, fusion procedures were performed at all levels with ruptured PLC to minimize potential segmental instability. Even with solid posterior fusion, deformity may occur and deteriorate when the disc narrows, suggesting that the long term stability of the FSU is doubtful when a non-fusion method is used [21]. In such situations, we recommend circumferential fusion (especially strut grafting in the disc space [24]) and implant preservation.
Fusion strategy is also determined by neurological status. For patients with complete or severe loss of neurological function (Usually ranked C or worse by ASIA scale, whole fusion is recommended since it is more important to fulfill stability rather than mobility. In addition, the fact that the integrity of PLC is usually destroyed when decompress to save spared neural function strengthens the use of fusion. For patients with minimal or no neural dysfunction, mobility of the spine is of importance for returning to ADL, thus selective or non-fusion are preferred to whole fusion.
Based on the abovementioned strategy, less than 10% of patients underwent non-fusion surgery, more than 50% underwent selective fusion and about 40% underwent whole fusion. The overall clinical outcome of those with normal neurological status is satisfactory, while stiffness is a major complaint especially in whole fusion patients, although may not affect their daily activities. The instrumentation and fusion seems to have limited impact on ADL because the mobility of the thoracolumbar junction is less important for ADL than that of the lumbar spine. However, it is convincing that non-fusion and selective fusion procedures have better long term outcomes since more mobile segments is preserved and less risk of adjacent level disease is taken, although a study with larger samples and long term follow-up should be conducted to corroborate this hypothesis.
Present study clearly shows that individualized fusion strategy should be based on the integrity of the vertebral endplate and PLC as well as severity of neural dysfunction in treatment of thoracolumbar fracture.
The present study has been conducted by the approval from the ethics committee of the Third Military Medical University in December, 2003 and all the informed consents to participate in the study have been obtained from participants.
Not applicable.
Not applicable.
The authors declare no competing financial interests and consent for publishing.
This project was funded in part by National Natural Science Fund of China (81401016 and 81171718).
Dr. Mingyong Liu conceived of the study, provided financial support and drafted the manuscript. Drs. Weili Fan, Xiang Ying, YaoyaoLiu, and Liangmin Zhang participated in the clinical data collection and clinical follow-up. Drs. Peng Liu and Jianhua Zhao supervised the conduction of the study and reviewed the manuscript. All authors read and approved the final manuscript.
None.