The treatment of perianal fistulas currently presents a major challenge to colorectal surgeons. In the present study, we consider how the type of curettage performed may influence the outcome, with respect to sealing the perianal fistula.
We present a retrospective study of 65 patients operated on for perianal fistula, sealed with platelet-rich fibrin (PRF). The patients were divided into two groups. Prior to PRF application, the patients in Group A underwent curettage by traditional methods. For those in group B, the curettage was performed using a graduated set of cylindrical curettes.
The groups were homogeneous in terms of sex, ASA classification, type of fistula, morbidity and postoperative incontinence. The only important difference recorded was in the rate of closure, which ranged from 52.9% with the use of traditional curettes to 80.6% with the set of cylindrical curettes (p = 0.03).
The results obtained lead us to believe that, for patients with perianal fistula, using a graduated set of cylindrical curettes to debride the tract obtains better results than conservative treatment, with no ill effects on postoperative sphincter continence.
Curettage, Anal fistula, Sealant
Perianal fistula is a common disorder that produces a strong negative impact on patients' quality of life. The aim of fistula surgery is to eradicate the fistula tract by closing the internal orifice and to do so without endangering continence. Simple open fistulotomy, which is commonly used for low fistulas, is often unsuitable for complex ones, as it bears a significant risk of impairing continence. Various procedures have been suggested for treating complex perianal fistulas, but none have yet gained wide acceptance [1]. In recent years, with the objective of avoiding damage to the sphincter in treating this type of fistula, numerous techniques based on sealing the tract have been proposed, using materials such as fibrin, stem cells or bioprosthetic plugs [2-7].
In 2009, our hospital began to use PRF (which can accelerate tissue growth) for the treatment of vascular ulcers of the lower limbs. This produced very good results, and so in 2011 we considered the possibility of using this substance to seal perianal fistulas (in the view that a fundamental treatment goal is to accelerate and enhance healing within the fistulous tract). Accordingly, a multicentre study was undertaken, the results of which were published in 2015 [8].
We then considered how these results could be improved. PRF accelerates tissue healing, but the tract contains fibrous matter that sometimes does not allow tissue to grow within it. This situation is similar to what happens if we seek to join the fibrous edges of a wound; in these circumstances, the wound will heal poorly, and so we must previously resect the fibrosis (by the Friedrich technique), thus obtaining healthy edges that will be able to heal properly. In this regard, several studies have highlighted the importance of resecting fibrous tissues in order to obtain good treatment results for anal fistulas [9,10].
Taking into account these previous studies, and convinced of the need to perform a complete curettage of the tract for optimum treatment results, in 2012 we designed a graduated set of cylindrical curettes that could be passed through the fistulous tract to debride all the fibrous tissue within it (Figure 1). In the present study, we compare the results obtained by PRF sealing using traditional curettage vs. the use of these cylindrical curettes.
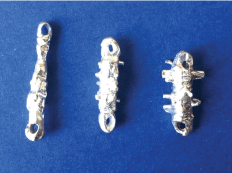 Figure 1: Set of cylindrical curettes, in three different sises.
View Figure 1
Figure 1: Set of cylindrical curettes, in three different sises.
View Figure 1
We present a retrospective study of all the patients at our hospital who underwent perianal fistula sealing with PRF between February 2011 and June 2016 (n = 65). The study was endorsed by the research, bioethics and biosafety committees and all the patients signed an informed consent. Two groups of patients were compared: group A (n = 34) was composed of patients treated with curettage of the tract by traditional methods (Volkmann spoon, endobrush and gauze strips with hydrogen peroxide); for those in group B (n = 31), the curettage was performed using a purpose-designed graduated set of cylindrical curettes.
Traditional curettage consists of applying a Volkmann spoon to the internal and external orifices, an endobrush in the area proximal to these orifices and gauze strips soaked in hydrogen peroxide along the tract.
The alternative approach we consider is to use a set of graduated, cylindrical curettes (Figure 1) which are small enough to be passed along the entire fistula tract. Each such curette has a hole at the extremities through which sutures are tied; these are then passed through the fistulous orifice, which allows us to apply traction at both ends of the curette, thus drawing it from one end to the other of the tract as many times as necessary (Figure 2). This study included all patients treated at our hospital for single-tract trans-sphincteric and supra-sphincteric perianal fistulas, and also those with inter-sphincteric fistulas with sphincter dysfunction (diagnosed by clinical history, anal examination, endoanal ultrasound and manometry).
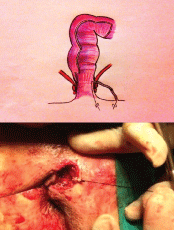 Figure 2: Curettage of the fistula tract using traction applied to the curette by the two sutures.
View Figure 2
Figure 2: Curettage of the fistula tract using traction applied to the curette by the two sutures.
View Figure 2
The following patients were excluded from the study: those with Crohn's disease, acute inflammatory process or complex perianal fistulas with multiple fistulous tracts and cavities, and also those who had received previous treatment for perianal fistula with biological sealing on two or more occasions.
The patients were admitted to hospital at least two hours prior to the intervention for the necessary preparation (blood extraction and processing and fibrin preparation) to be performed.
The surgical technique applied is quite simple, and involves the following steps:
1. Channel the tract with a grooved probe.
2. Resect the fibrous areas of the internal and external orifices.
3. Curette the tract to create a raw surface (Group A by the traditional method and Group B with the set of small curettes).
4. Wash with hydrogen peroxide, since active bleeding impedes the action of growth factors.
5. Seal the tract, using an applicator unit, which is fitted with a monitor showing the amount of product remaining.
6. Close the internal orifice.
To evaluate the results obtained, each case was reviewed in outpatient consultation, at one week, three months, six months, one year and two years and (by telephone) at the conclusion of the study. The fistula was considered to have healed if there was no spontaneous, pressure-induced drainage through the external orifice and if it was completely epithelialised. If closure was not achieved during the six months following the intervention, the treatment was considered unsuccessful.
Anal continence was measured by the Wexner test, which was applied before and after the intervention in order to determine the difference between the two values obtained. To maximise the response rate, the few patients who did not come to the hospital for a scheduled review were contacted by telephone.
The two groups were evaluated taking into account the following variables: Sex, ASA classification, type of fistula, morbidity, need for a second application, need for second surgical sealing, fistula closure and difference between pre- and postoperative Wexner test scores.
To determine whether the differences observed in the frequencies of the study variables were statistically significant, the qualitative variables were evaluated by the chi-square test, and by Fisher's exact test for the 2 × 2 tables or if more than 20% of the expected values were less than 5. To analyse the differences between continuous quantitative variables in two independent groups, Student's t test was applied to two independent samples, after verifying the normal distribution of the variables in each group (by the Shapiro-Wilk test). When the distribution was non-normal, the corresponding non-parametric Mann-Whitney U test was applied.
The two groups were homogeneous in terms of age, sex, ASA classification, type of fistula and duration of follow-up (mean follow-up period was 29.29 months for group A and 27.32 months for group B).
As shown in Table 1, there were no significant differences between the groups in terms of morbidity (one case of mild perianal infection in group A, one case of abscess in group B), the need for a second intervention for resealing or for a second application of PRF in outpatient consultation or in the time elapsed until the closure of the fistula.
Table 1: Results of the compared parameters. View Table 1
Fistula closure was achieved in 52.9% of the patients in group A vs. 80.6% of those in group B (p = 0.03).
There were no significant differences in the Wexner pre- and post-intervention test results (no change in group A and minor changes in two cases in group B, of one and three points respectively).
This study was undertaken to determine whether the use of our purpose-designed set of graduated curettes produces better results than traditional methods in the treatment of perianal fistula by PRF sealing. The problem of perianal fistula is more acute when the tract is high, because then the treatment is necessarily complex (limited surgery can lead to recurrence, whereas aggressive surgery is associated with higher rates of faecal incontinence). Therefore, when deciding on the most appropriate treatment, the expected success rate should be weighed against the risk of sphincter injury and of reduced continence, which can have a long-term detrimental effect on the patient's quality of life. A recent study concluded that most patients preferred sphincter preservation techniques even at the risk of poorer results [11]. In other words, patients attributed greater importance to reducing the risk of incontinence than to achieving a higher rate of healing.
In recent years, various approaches have been proposed for the conservative treatment of fistulas that affect a large volume of fibres within the sphincter apparatus, based on sealing the fistula with materials such as fibrin [12-14], stem cells [6,15,16] or bioprosthetic plugs [4,17,18].
In all of these conservative techniques for the treatment of perianal fistula, it is accepted that the tract must be curetted before applying the corresponding preparation, although the importance of this step may not always be emphasised.
The results obtained from the treatment of complex fistulas may be directly proportional to the amount of fibrous tissue resected from the tract. If this were so, outcomes would be improved if complete curettage of the fistula tract could be achieved.
In accordance with this hypothesis, we manufactured a set of cylindrical curettes [19], first as a basic prototype and later as a more sophisticated model, using a 3D printer (Figure 3). Each curette presented spiculated areas on the surface so that as it passed through the tract, the fibrous tissue of the fistula would be resected. In designing these curettes, it was necessary to determine the quantity of fibrous tissue we needed to resect. Therefore, we examined 50 pelvic MRI scans of patients with perianal fistula to evaluate the diameter of fibrous tissue within the tract. In 95% of the cases, this diameter was less than 5 mm, and so we manufactured a graduated set of curettes, with the largest measuring 5 mm, thus ensuring that by successively passing these curettes through the tract, all the fibrous tissue would be resected in at least 95% of the cases.
 Figure 3: Set of cylindrical curettes (model created using a 3D printer).
View Figure 3
Figure 3: Set of cylindrical curettes (model created using a 3D printer).
View Figure 3
The standard surgical technique (to insert the PRF plug) would be reinforced by debriding the fistula tract to remove the granulation tissue from the entire tract [20], thus improving the presence of healthy tissue and blood flow, as recommended in studies of experimental models in pigs [9,10].
Our study corroborates this approach. When the cylindrical curettes produced a better curettage of the tract, the results obtained were considerably improved, with rates of fistula closure rising from 52.9% to 80.6% (p = 0.03). It should be taken into account that traditional methods for debriding the fistula tract include the injection of hydrogen peroxide, the application of saline solution, and curettage with an endobrush or curette, but none actually remove the fibrous tissue from the tract.
In our study, the Wexner questionnaire [21] was used to evaluate pre- and post-operative continence. Overall, the results in this respect did not vary significantly in either of the two groups. Thus, there was no variation in group A, while in group B changes were only observed in two patients, whose Wexner scores rose by one and three points out of 20 following the intervention.
In conclusion, the data obtained in this study lead us to believe that, in most cases, the use of our set of graduated cylindrical curettes removes all the fibrous tissue from the tract, thus achieving significantly better results than the techniques currently used in the treatment of complex fistulas, without any negative impact on outcomes regarding anal continence and morbidity and mortality.
We are grateful to Rita Pérez (FIMABIS) for the support with the statistical analysis.
Francisco Javier Pérez Lara and other co-authors have no conflicto of interest.
FJ Pérez Lara: Made a substantial contribution to the concept and design, drafted the article or revised it critically for important intellectual content, approved the version to be published.
JM Hernández González: Approved the version to be published.
A Ferrer Berges: Approved the version to be published.
H Oehling de los Reyes: Approved the version to be published.
H Oliva Muñoz: Approved the version to be published.