International Journal of Surgery Research and Practice
Vancomycin and Imipenem Release from Nails Covered with Antibiotic-Loaded Acrylic Cement
Jorge D Barla, Sancineto F Carlos, Luciano A Rossi*, Gimenez I Maria, Visus M and Elizondo Cristina
Italian Hospital from Buenos Aires, Peron 4190 (C1199ABB) Buenos Aires, Argentina
*Corresponding author: Luciano Andres Rossi, Italian Hospital from Buenos Aires, Peron 4190 (C1199ABB) Buenos Aires- Argentina, Tel: 1-558733374, E-mail: luciano.rossi@hospitalitaliano.org.ar
Int J Surg Res Pract, IJSRP-2-031, (Volume 2, Issue 2), Research Article; ISSN: 2378-3397
Received: July 25, 2015 | Accepted: November 14, 2015 | Published: November 17, 2015
Citation: Barla JD, Carlos SF, Rossi LA, Maria GI, Visus M, et al. (2015) Vancomycin and Imipenem Release from Nails Covered with Antibiotic-Loaded Acrylic Cement. Int J Surg Res Pract 2:031. 10.23937/2378-3397/1410031
Copyright: © 2015 Barla JD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: There is a lack of information in the literature regarding pharmacokinetic properties of nails covered with antibiotic-loaded acrylic cement. The aim of this research work was to describe the release of vancomycin and imipenem from nails covered with ALAC over a period of 6 weeks. Furthermore, we analyzed if an increased nail diameter associated to a thicker ALAC coat could result in an increased antibiotic elution from the cement and if the combination of the two antibiotics in the same cement affects the amount of antibiotics released.
Materials and Methods: Two groups of three nails each were defined. Group 1: consisting of three 10 mm nails covered with cement with a final diameter of 14 mm. Nail 1: Simplex cement with tobramycin + 4 g of vancomycin per 40 g of cement. Nail 2: Simplex cement with tobramycin + 2 g of imipenem per 40 g of cement. Nail 3: Simplex cement with tobramycin + 4 g of vancomycin + 2 g of imipenem per 40 g of cement. Group 2: we used the same antibiotics but the three 10 mm nails were covered with cement with a final external diameter of 16 mm.
Results: In vitro concentrations of vancomycin and imipenem maintained above the MIC for at least 6 weeks. Increasing the external diameter of the nail did not modified the concentration of antibiotics released significantly (p = 0.481). The release of the antibiotics was not significantly modified by the combination of vancomycin and imipenem in the same nail (p = 0.38).
Conclusions: It is possible to achieve in vitro concentrations of vancomycin and imipenem above the MIC for at least 6 weeks with nails covered with antibiotic-loaded acrylic cement. Neither the combination of both antibiotics nor the increase in the diameter of the nail significantly modified the release of the antibiotics.
Keywords
Antibiotic-loaded cement, Antibiotics imipenem, Vancomycin
Introduction
Antibiotic-loaded acrylic cement (ALAC) was first introduced by Buchholz and Engel Brecht [1] in 1970 for the treatment of infections in orthopedics and orthopedic surgery, and its use is currently still in force. ALAC enables larger local antibiotic concentrations in soft and osseous tissues than those enabled by systemic administration, especially in tissues with poor blood circulation [2]. On the other hand, low serum concentrations of antibiotic reduce its systemic toxicity. Previous researches on the release of antibiotics from intramedullary implants have shown encouraging results [3-5]. Vester et al. used both laboratory models and rat tibias to show the capacity of titanium implants covered with gentamicin for achieving high concentrations of antibiotic with no bacterial resistance, and without affecting the normal functioning of osteoblasts [3]. There are several studies available that have attempted to define the kind of antibiotic, its ideal concentration and the best cement [6-9].
Historically, ALAC has been used in the shape of beads to treat infections [10]. As these do not provide mechanical stability, in the case of non-consolidated fractures, they must be associated with some method of osteosynthesis. One form of associating local antibiotic release with mechanical stability has been the development of intramedullary nails covered with ALAC. Whether this nail is used as a definitive treatment or as a temporary stabilization system within the reconstruction of an osseous segment, it is essential to know the diffusion dynamics of the antibiotic towards its environment, its concentration and the period of time during which its bactericidal action remains effective. This has been studied with bead-shaped ALAC [11].
Even though there are reports of positive clinical results associated with nails covered with cement, we could not find in the scientific literature any information related to the release time and antibiotic concentrations around these implants [12-15]. Moreover, in these different publications, the production method for nails with ALAC was not homogeneous. In this project, we will employ a protocol already described by the authors [15].
The aim of this research work was to describe the release of vancomycin and imipenem from ALAC coated nails over a period of 6 weeks and to assess if levels remain above the minimum inhibitory concentration (MIC) for those bacteria most frequently involved in infections of the large bones. We analyzed as well if an increased nail diameter associated to a thicker ALAC coat could result in an increased antibiotic elution from the cement. Furthermore, we searched if the combination of the two antibiotics in the same cement affects the amount of antibiotics released.
Materials and Methods
Preparation of nails with ALAC
To build-up each device, astainless-steel-520 10 mm in diameter and 300 mm long nail, a plastic tube 14 mm in internal diameter × 300 mm long or a second tube 16 mm × 300 mm long, and two units of Simplex Bone Cement with tobramycin (Howmedica International, London, United Kingdom) were used as materials in this study.
The cement powder and the antibiotic powder were mixed for 1 minute in a reservoir. Next, the liquid component of the cement was added and mixed again. While the cement was still fluid, it was compressed into the plastic tube. The nail was inserted into the tube. Once the cement had polymerized, the plastic tube was cut and the nail covered with cement was ready to use.
Depending on which antibiotic was added to the cement, two groups of three nails each were defined.
Group 1: Consisting of three 10 mm nails covered with cement with a final diameter of 14 mm.
Nail 1: Simplex cement with tobramycin + 4 g of vancomycin per 40 g of cement.
Nail 2: Simplex cement with tobramycin + 2 g of imipenem per 40 g of cement.
Nail 3: Simplex cement with tobramycin + 4 g of vancomycin + 2 g of imipenem per 40 g of cement.
Group 2: Consisting of three 10 mm nails covered with cement with a final external diameter of 16 mm.
Nail 1: Simplex cement with tobramycin + 4 g of vancomycin per 40 g of cement.
Nail 2: Simplex cement with tobramycin + 2 g of imipenem per 40 g of cement.
Nail 3: Simplex cement with tobramycin + 4 g of vancomycin + 2 g of imipenem per 40 g of cement.
Measurements
Once they had been prepared, each nail was inserted into a glass tube filled with saline solution at 37°C and protected from sunlight until sampling. At different intervals, the nail was extracted from the tube and 5 ml of saline solution were collected in a sterile fashion. These samples were frozen at -20°C until the time of measurement.
The nails were cleaned with 0.9% sodium hydrochloride solution and were placed into glass tubes with fresh saline solution.
Samples were taken at 1, 2, 4, 8 and 24 hours on the first day, then daily from the second to the seventh day, and finally weekly until the sixth week.
Assessment
The amount of antibiotic released during each stage was measured by means of mass spectrometry using the UPLC Acquity Ultra Performance LC Xevo TQ MS equipment (Figure 1).

.
Figure 1: UPLC Acquity Ultra Performance LC XevoTQ MS. Equipment used for measuring antibiotic concentrations by mass spectrometry.
View Figure 1
The antibiotic concentration release was expressed in μg/ml. The total amount of antibiotic released by each nail at each time point was measured by multiplying the concentration/ml by the amount of saline solution in each tube. The antibiotic release rate (μg/ml) from each nail was obtained by dividing the total amount of antibiotic released by the release time (in hours).
The release of vancomycin from the nails that contained that antibiotic was measured first. Secondly, the release of imipenem was measured from the nails that contained this antibiotic, and, lastly, it was determined if there was any significant difference between the released amounts.
After the measurement, four major variables were analyzed:
Firstly, the release of vancomycin from the nails containing that antibiotic (ATB) in the cement was measured for 6 weeks. The aim was to assess if the ATB concentrations remained above the MIC of the most frequent bacteria involved in osteomyelitis, i.e. coagulase-negative staphylococci (CNS) and Staphylococcus aureus (SA). The MIC values are 4 μg/ml and 2 μg/ml, respectively.
Secondly, the release of imipenem from the nails containing that antibiotic in the cement was measured for 6 weeks. The aim was to assess if ATB concentrations remained above the MIC of Pseudomonas aeruginosa (PSA) as a representative of Gram-negative bacilli; the MIC is 2 μg/ml.
Thirdly, it was studied whether the combination of the antibiotics or an increase in the diameter of the nail at the expense of its preparation with a larger quantity of cement altered the pharmacokinetics of the antibiotic release.
Statistical analysis
The differences in antibiotic release over time were analyzed by means of linear regression, taking into account the natural grouping of the measurements in each nail; the diameter of the nail (14 mm vs. 16 mm) and the combination of both antibiotics in the same nail were taken into account as additional explanatory variables. The data were analyzed using STATA software, version 12 (Stata Corporation, College Station, Texas, USA). P < 0.05 was significant; additionally, the elimination rates and their 95% confidence intervals are presented.
Results
Regarding the first point under evaluation, we observed that all four nails containing vancomycin in the cement released amounts above the MIC of CNS and SA over the period of 6 weeks (Figure 2).
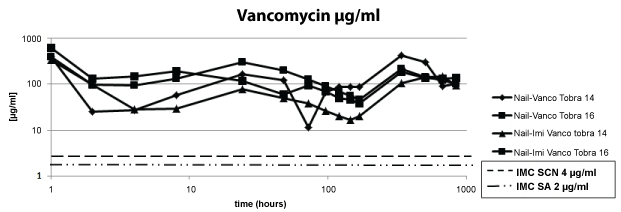
.
Figure 2: The concentration of vancomycin remained above the MIC over the period of 6 weeks.
View Figure 2
Regarding the second point under evaluation, we observed that all four nails containing imipenem in the cement released concentrations above the MIC of PSA over the period of 6 weeks (Figure 3).
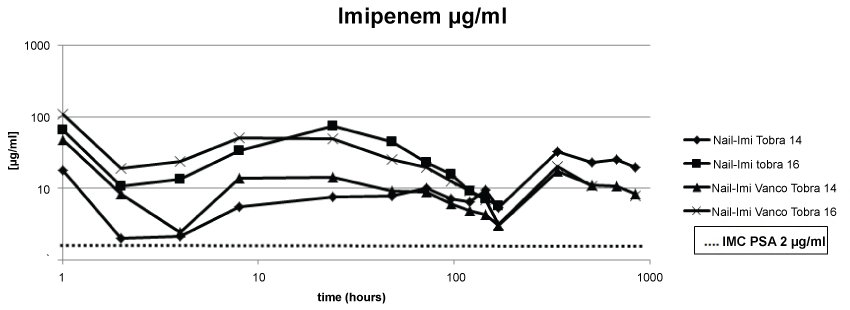
.
Figure 3: The concentration of imipenem remained above the MIC for PSA over the period of 6 weeks.
View Figure 3
Time was the only variable which significantly modified the release of antibiotics, even when adjusted for the size of the nail and the combination of antibiotics in the cement.
Regarding the pharmacokinetics of the release of antibiotics when increasing the diameter of the nail, we found that by the sixth week, effective concentrations were still being released, but they were not significantly greater than those released with a smaller amount of cement. The same occurred when we observed the elution of the antibiotic with the combination of vancomycin and imipenem in the same nail; the release of the antibiotics was not significantly modified when compared to the concentrations achieved by the nails which contained only one of them (Table 1).
![]()
Table 1: Neither the combination of antibiotics, nor the increase in the diameter of the nails, resulting in the use of more cement, significantly modified the release of antibiotics.
View Table 1
Discussion
Local antibiotic therapy using antibiotic-loaded surgical cement beads has proven to be useful in the treatment of osteomyelitis [16-18]. Many studies have shown that the combination of local antibiotics with intravenous therapy allows for better control of the infection [19,20]. On the other hand, in osteomyelitis, the scientific literature shows fracture stability to be extremely important in order to achieve positive results, especially in infected non unions [21-24].
Intramedullary nails covered with antibiotic-loaded surgical cement have certain benefits in the treatment of bone infections, which explains why the use of these materials has expanded in the last decade [21-25]. In the first place, the reaming of the endomedullary canal is a valuable complement to achieving full initial debridement. Secondly, such reaming allows for preparing the canal for the insertion of a final locking nail, if no consolidation was achieved after the initial local antibiotic therapy.
In the clinical scenario, the use of intramedullary cement nails with antibiotics has been previously described in the scientific literature by several authors [16,26,27]. Paley and Herzenberg [25] provided preliminary results on the treatment of nine patients. All patients but one in their series had acute osteomyelitis associated with lengthening of the limb by means of external fixation through intramedullary nails. No patient showed signs of recurrence during the monitoring phase. In a more recent study, Thonse and Conway [13] presented their clinical experience with intramedullary nails covered with antibiotic-loaded cement. The authors concluded that the additional stability given by the nails in addition to local antibiotic therapy is a good alternative in the treatment of long bone infections.
In our previously published series, [15] we also obtained satisfactory results from 19 consecutive patients treated for a minimum of 12 months for osteomyelitis of long bones with intramedullary nails covered with antibiotic-loaded cement. Nevertheless, in spite of positive clinical results regarding the elimination of the infection, there is not enough information in the about the pharmacokinetics of the release of different antibiotics form this type of implants.
For the present study, we chose vancomycin and imipenem (since these are the antibiotics most commonly used in our field) together with tobramycin (since it is already incorporated in the cement) because they are the most effective antibiotics against the common causative organisms of bone infections.
Many antibiotics have been tested together with polymethyl methacrylate [9,16]. The antibiotics used for this study have been shown to achieve effective concentrations for up to 6 weeks when administered locally. Still, previous studies mostly focused on the release from pearls or cement spacers [2,19,20,28] and not from covered nails. We believe that the usefulness of this research is that it shows that both vancomycin and imipenem maintain effective concentrations in vitro up to 6 weeks, a period of time that is usually described for the intravenous treatment of acute infections, whether they are used individually or combined, as it may be necessary in the case of a polymicrobial infection. Although the antibiotic elution was followed up to 6 weeks, figures show that the antibiotic concentration was still way above the MIC and leads to think that these nails may still be effective during a longer period of time from what was previously described. Finally, we consider it to be a relevant fact that an increase in the diameter of the cement covering with antibiotics was not followed by a significant increase in the released concentration of antibiotics.
The main limitations of the present research are, considering that this was an in vitro investigation; it is not possible to measure some pharmacokinetic modifications that might occur in patients as a result of antibiotic metabolism. Nevertheless, we believe that given the difficulties inherent in conducting this type of study on humans, and given the relevancy and increase in infections in recent years, it is essential to investigate the pharmacokinetic behavior of various antibiotics released from local devices.
In conclusion, the present research shows that, using nails covered with ALAC, it is possible to achieve in vitro concentrations of vancomycin and imipenem above the MIC for at least 6 weeks. Neither the combination of both antibiotics nor the increase in the diameter of the nail significantly modified the release of the antibiotics. The positive clinical results achieved by means of the stabilization of infected non unions using a combination of cement-covered nails and antibiotics makes it necessary to further study for how long the release of antibiotics from the cement remains effective (MIC). Long bones with infected non unions probably need more than 6 weeks to achieve consolidation. Keeping the same implant to enhance mechanical stability competes with the possibility of recurrence of the infection. Further studies are required in order to determine the duration of effective local treatment.
Acknowledgement
We would like to acknowledge to AOLAT (AO foundation Latin America) for the support in the development of this study.
References
-
Buchholz HW, Engelbrecht H (1970) Depot effects of various antibiotics mixed with Palacos resins. Chirurg 41: 511-515.
-
Cerretani D, Giorgi G, Fornara P, Bocchi L, Neri L, et al. (2002) The in vitro elution characteristics of vancomycin combined with imipenem-cilastatin in acrylic bone-cements: a pharmacokinetic study. J Arthroplasty 17: 619-626.
-
Vester H, Wildemann B, Schmidmaier G, Stockle U, Lucke M (2010) Gentamycin delivered from a PDLLA coating of metallic implants: In vivo and in vitro characterisation for local prophylaxis of implant-related osteomyelitis. Injury 41: 1053-1059.
-
Lucke M, Schmidmaier G, Sadoni S, Wildemann B, Schiller R, et al. (2003) Gentamicin coating of metallic implants reduces implant-related osteomyelitis in rats. Bone 32: 521-531.
-
Mader JT, Stevens CM, Stevens JH, Ruble R, Lathrop JT, et al. (2002) Treatment of experimental osteomyelitis with a fibrin sealant antibiotic implant. Clin Orthop Relat Res: 58-72.
-
Kuechle DK, Landon GC, Musher DM, Noble PC (1991) Elution of vancomycin, daptomycin, and amikacin from acrylic bone cement. Clin Orthop Relat Res: 302-308.
-
Penner MJ, Duncan CP, Masri BA (1999) The in vitro elution characteristics of antibiotic-loaded CMW and Palacos-R bone cements. J Arthroplasty 14: 209-214.
-
Elson RA, Jephcott AE, McGechie DB, Verettas D (1977) Antibiotic-loaded acrylic cement. J Bone Joint Surg Br 59: 200-205.
-
Hughes S, Field CA, Kennedy MR, Dash CH (1979) Cephalosporins in bone cement: studies in vitro and in vivo. J Bone Joint Surg Br 61: 96-100.
-
Adams K, Couch L, Cierny G, Calhoun J, Mader JT (1992) in vitro and in vivo evaluation of antibiotic diffusion from antibiotic-impregnated polymethylmethacrylate beads. Clin Orthop Relat Res: 244-252.
-
Luksa J, Marusic A (1995) Rapid high-performance liquid chromatographic determination of vancomycin in human plasma. J Chromatog B Biomed Appl 667: 277-281.
-
Selhi HS, Mahindra P, Yamin M, Jain D, De Long WG Jr, et al. (2012) Outcome in patients with an infected nonunion of the long bones treated with a reinforced antibiotic bone cement rod. J Orthop Trauma 26: 184-188.
-
Thonse R, Conway J (2007) Antibiotic cement-coated interlocking nail for the treatment of infected nonunions and segmental bone defects. J Orthop Trauma 21: 258-268.
-
Bhadra AK, Roberts CS (2009) Indications for antibiotic cement nails. J Orthop Trauma 23: S26-30.
-
Sancineto CF, Barla JD (2008) Treatment of long bone osteomyelitis with a mechanically stable intramedullar antibiotic dispenser: nineteen consecutive cases with a minimum of 12 months follow-up. J Trauma 65: 1416-1420.
-
Henry SL, Ostermann PA, Seligson D (1993) The antibiotic bead pouch technique. The management of severe compound fractures. Clin Orthop Relat Res: 54-62.
-
Ostermann PA, Seligson D, Henry SL (1995) Local antibiotic therapy for severe open fractures. A review of 1085 consecutive cases. J Bone Joint Surg Br 77: 93-97.
-
Zalavras CG, Patzakis MJ, Holtom P (2004) Local antibiotic therapy in the treatment of open fractures and osteomyelitis. Clin Orthop Relat Res: 86-93.
-
Miller ME, Ada JR, Webb LX (1989) Treatment of infected nonunion and delayed union of tibia fractures with locking intramedullary nails. Clin Orthop Relat Res: 233-238.
-
Patzakis MJ, Wilkins J, Wiss DA (1986) Infection following intramedullary nailing of long bones. Diagnosis and management. Clin Orthop Relat Res: 182-191.
-
Tandon SC, Thomas PB (1996) Persistent osteomyelitis of the femur--2 cases of exchange intramedullary nailing with gentamicin beads in the nail. Acta Orthop Scand 67: 620-622.
-
Court-Brown CM, Keating JF, McQueen MM (1992) Infection after intramedullary nailing of the tibia. Incidence and protocol for management. J Bone Joint Surg Br 74: 770-774.
-
Klemm KW (1993) Antibiotic bead chains. Clin Orthop Relat Res: 63-76.
-
Klemm KW (1986) Treatment of infected pseudarthrosis of the femur and tibia with an interlocking nail. Clin Orthop Relat Res: 174-181.
-
Paley D, Herzenberg JE (2002) Intramedullary infections treated with antibiotic cement rods: preliminary results in nine cases. J Orthop Trauma 16: 723-729.
-
Ohtsuka H, Yokoyama K, Higashi K, Tsutsumi A, Fukushima N, et al. (2002) Use of antibiotic-impregnated bone cement nail to treat septic nonunion after open tibial fracture. J Trauma 52: 364-366.
-
Madanagopal SG, Seligson D, Roberts CS (2004) The antibiotic cement nail for infection after tibial nailing. Orthopedics 27: 709-712.
-
Ueng SW, Wei FC, Shih CH (1999) Management of femoral diaphyseal infected nonunion with antibiotic beads local therapy, external skeletal fixation, and staged bone grafting. J Trauma 46: 97-103.