Gut microbiota plays an important role in the modulation of physiological processes associated with the digestion of nutrients, immune system and control of energy homeostasis. Changes in gut microbiota composition have been associated notably with obesity, diabetes, and inflammatory diseases. Diet is one of the major factors capable of modulating the intestinal microbiota composition. In addition, the literature has shown that exercise can affect the gut microbiota composition and modulate the balance between the interaction of host and beneficial microbiota. Physical exercise improves the diversity and relative amounts of bacterial species under different nutritional contexts. However, the impact of exercise associated or not with dietary changes on the gastrointestinal environment and consequences for gut health remain poorly understood. Some proposals regarding the biological mechanisms possibly involved highlight the short chain fatty acid production and alteration in intestinal pH as main forms by which exercise may affect gut microbiota composition. Thus, the aim of the present review is to present an overview of the effects of physical exercise associated with diet on the characteristics of the intestinal microbiota.
Exercise, Diet, Gut microbiota, Immune system, Short chain fatty acid
Gut microbiota is now established as a key player in various aspects of health and diseases [1]. Recently, commensal bacteria have been shown to be able to affect gut metabolism and physiology by several mechanisms, including the production of various bacterial metabolites from dietary and endogenous substrates [2]. While carbohydrate fermentation is mainly considered beneficial for the host through the production of Short-Chain Fatty Acids (SCFA) in the intestinal luminal content, protein fermentation gives rise to a wide variety of compounds, some of which could be detrimental for gut health when present at excessive concentration [3]. Some bacterial metabolites can be transferred through the intestinal epithelium from the intestinal luminal content to the portal bloodstream reaching the liver, and then, to the peripheral blood stream [4]. Some of these metabolites have been shown to be active on different tissues, such as in the liver and adipose tissue, by interfering with metabolism and physiology [5]. In addition, high fat diet consumption may be capable of promoting gram-negative bacteria growth and favoring a local inflammation, which would be harmful for gut health [6]. There are several reports regarding the effects of the diet but, recently, exercise was revealed as another factor capable of influencing the diversity, composition and metabolic activity of gut microbiota, as well as its fermentation capacity and diet SCFA production [6,7]. The aim of the present review is to present an overview of the effects of physical exercise associated with diet on the characteristics of the intestinal microbiota.
The human gastrointestinal tract is colonized by approximately one trillion microorganisms known as gut microbiota. This diversity represents a number much larger than human cells [8,9]. Bacterial density varies along the gastrointestinal tract due to the specific conditions of each portion, such as differences in the gradient of pH, antimicrobial peptides (including bile acids), and in the amount of oxygen, which limits the growth of some bacteria [10].
Human gut microbiota composition varies since the birth up to two years of age, when the birth delivery by vaginal or cesarean mode and early nutrition by breastfeeding or formula milk and the introduction of new food modulate initially the microbial populations, quantitatively and qualitatively, of the child toward adulthood [11,12]. From there, several environmental factors, mainly diet, exercise, aging, hygiene, medicine, geographic area, pregnancy and the presence or not of some disease will influence the microbial composition in a host-specific way [13,14]. In this complex community of bacteria, two phyla appear to be the most predominant and common among individuals: Firmicutes (60-80%) and Bacteriodetes (15-30%) [15]. The first one is the most abundant phylum covering, mainly, Clostridium, Ruminococcus, Lactobacillus and the butyrate-producing bacteria, such as Eubacterium, Faecalibacterium and Roseburia, which are known for their abundance in healthy individuals. The Bacteroidetes phylum is composed, primarily, by gram-negative bacteria, including Bacteroides genus, which is recognized, mostly, for its contribution to the degradation of complex glycans [16]. Furthermore, there are others phyla, which are part of the gut microbiota, but in minor proportion, such as Proteobacteria, Verrucomicrobia, Actinobacteria, Fusobacteria and Cianobacteria [17].
Diet influences gut microbiota composition since it provides senergy, nutrients and oligoelements/micronutrients, which will be used by both the host and intestinal bacteria. The gut microbiota produces several vitamins and a range of enzymes, which will ferment the nutrients that are not digested by human digestive enzymes [18]. The most abundant SCFAs produced by fermentation of carbohydrates are acetate, propionate and butyrate (which constitute > 95% of the SCFA content). It has been shown that butyrate acts locally on intestine by affecting metabolism and gene expression in the colonic epithelium [19,20] while acetate and propionate reach systemic circulation and are utilized by other organs, such as adipose tissue and liver, and contribute up to 10% of the energy required by the host [21]. Moreover, as weak acids, they also help to maintain a slightly acidic pH in the proximal colon.
Nondigested proteins or peptides might also be substrates for microbial production of SCFA [2]. However, microbial protein fermentation by proteolytic bacteria (for example some bacteria of Clostridium's group) yields a diverse range of metabolites, including Branched-Chain Fatty Acids (BCFA), lactate, and aromatic components, and amines, sulfides, phenols and indoles [22]. Many of these protein fermentation-derived metabolites might have negative consequences on epithelial cell metabolism and barrier function, affecting the host's gut health [23]. Moreover, high protein diets are usually accompanied by a reduction in carbohydrate intake, which may not be beneficial for host health [24]. Currently, little is known about the effects of protein supplementation, associated (or not) with exercise.
Animal fat-rich diets quickly increase the abundance of bacteria resistant to bile acids in humans, such as Bacteroides and Bilophila, which can metabolize different types of bile acids and promote the development of inflammatory bowel disease [25]. Furthermore, consumption of a high fat diet is capable of unbalancing the proportions of Firmicutes/Bacteroidetes, raising Lipopolysaccharide (LPS) circulation and the concentration of inflammatory cytokines, favoring systemic inflammation [25,26].
Studies in humans reporting the effects of physical exercise on gut microbiota composition, diversity and metabolic activity are limited. Clarke, et al. [27] accomplished the sole study performed with healthy individuals. In this pioneering work, elite rugby players were recruited, and, in agreement with several animal studies, this study reported that athletes displayed an increase of the gut microbiota richness and diversity (22 distinct phyla), and also a decrease of systemic pro-inflammatory cytokines [27] (Table 1). The authors reported this biota profile in individuals with exercise training program in athletes as compared to sedentary controls group.
Table 1: Exercise and microbiota studies. View Table 1
However, the most relevant insight on the effect of exercise on gut composition was provided by experimental models. Matsumoto, et al. [28] were the first authors to demonstrate that chronic voluntary physical exercise is able to change the composition of rat gut microbiota [28]. Some studies performed afterwards associated physical exercise with some pathological states or dietary intervention (Table 1).
A study performed with polychlorinated biphenyls (pollutant model) demonstrated that voluntary physical exercise is able to cause changes in the biodiversity and composition of microbiota in mice, and attenuated the effects of the pollutant contamination of the microbiota [29]. Furthermore, under different dietary conditions, voluntary exercise appears to reshape the gut microbiota. Evans, et al. [25] proposed that physical exercise modifies the bacterial balance in the gut, with alteration of the major phyla levels, and increase of the relative proportion of butyrate-producing bacteria (Clostridiaceae, Lachnospiraceae and Ruminococcaceae). The authors appoint that the exercise practice would be able to prevent the effects of a High Fat Diet (HFD) [25]. In fact, Campbell, et al. [26] showed that exercise is able to modify not only the specific populations of commensal bacteria in the gut, but also cause morphological changes in gut microenvironment. In the Campbell et al. study [26], the exercised group showed reduced intestinal inflammation due to a high-fat diet and morphological characteristics similar to the control. Likewise, in a previous study, Faecalibacterium prausnitzi and Lachnospiraceae group (a Clostridia-cluster) were detected only in the group submitted to exercise, as we observe on Evans, et al. [25] research, hence supporting the hypothesis that exercise causes changes in gut microbiota independent of changes in diet [26].
Short term (6 days) voluntary exercise showed that nutritional status and physical activity alter gut microbiota diversity in different manners. When exercise is combinated with food restriction protocol (restricting access for 23 hours per day and confined to running wheels except during a 60 min meal), a negative impact on bacterial richness is reported with respect to the Lactobacillus and Bifidobacterium genera [30]. The caloric restriction was also able to modify the phyla, even in the presence of exercise [30] (Table 1). It seems that exercise cannot attenuate the effect of caloric restriction, whereas it would be able to cause improvements on gut microbiota composition even under high fat diets.
Voluntary exercise with different aerobic capacities, intensity, volume and frequency may present different outcomes. Evans, et al. [25] showed a significant increase in the abundance of Bacteroidetes, while the evidence provided by Liu, et al. [31] showed a reduction in the abundance of Firmicutes and Proteobacteria phylum. Apart from both studies exposing the animals to the same diet (HFD), these differences observed between these two studies may originate from the different experimental models used. Liu, et al. [31] study was performed with ovariectomized female rats fed with HFD, divided in High Capacity Running (HCR) and Low Capacity Running (LCR), performed 11 weeks of voluntary exercise whereas Evans, et al. [25] performed 12 weeks voluntary exercise in male rats [25,31].
Petriz, et al. [32] proposed that training status and intensity may be favorable to the proliferation of specific families of bacteria [32]. The authors reported an inverse correlation between exercise and Clostridiaceae/Bacteroideae families and Ruminococus genera, and a positive correlation between Oscillospira in exercise intensity. Aerobic training may be associated with a favorable environment for Clostridiaceae, Bacteroideae and Ruminococus, but an unfavorable environment for Oscillospira due to acidification of the intestinal environment. The proposition by Petriz, et al. [32] is in agreement with modification of the gut environment following high intensity or long period exercise. Exercise should modify the environment in a favorable way in terms of anaerobic bacteria population, or acidic environment since a decrease splanchnic blood flow and oxygen supply occurs [32].
A recent study performed using High Intensity Interval Training (HIIT), demonstrated that HIIT exerts opposed changes to the gut microbiota compared to those imposed by obesity profile. Indeed, HIIT reduces the predicted metabolic genetic capacity of the fecal microbiota, alters microbiota metabolic pathways, and raises the possibility that this type of exercise training may elicit some of its beneficial effects on metabolism through alterations in the gut microbiome [33]. However, this same study did not compare the different types of exercise, such as continuous versus intermittent, or voluntary versus controlled exercise. These parameters should be taken into consideration in future investigations, since it remains unclear if the different types of exercise can cause similar beneficial effects regarding gut microbiota, and intestinal health as suggested by Liu, et al. and Petriz, et al. [31,32].
Related to the beneficial impact of exercise on the microbiota composition and diversity in the early life, Mika, et al. [34] propose, "The sooner, the better". This proposition is related with the fact that microbiota is more plastic and sensitive to environmental changes during early life. Then, exercise initiated during the juvenile period may show a more robust impact on the gut microbiota than exercise initiated in adulthood [34]. In other words, the changes that occur in childhood may last longer even with the absence of exercise than the changes occurring later. The authors show that exercise-induced alterations in microbiota during early life contribute to metabolic consequences such as increased SCFA production, increased energy expenditure and reduced fat accumulation in the adipose tissue. Indeed, increased Bacteroidetes phyla along with decreased Firmicutes phyla within the gut have been associated with these metabolic consequences. The results obtained by Denou, et al. [33] reinforce this proposition [33-35].
It is important to observe that only one study regarding gut microbiota and exercise science was carried out with athletes [27], a population in which the amount of exercise training is very large and intense, and its outcomes are different when compared with voluntary exercise performed in some animals' protocols or short session of moderate exercise performed by active individuals, as shown in Table 1.
The effect of exercise on microbiota is still largely unknown. They are likely to be mediated, at least in part, by altering parameters that influence the intestinal microenvironment.
Exercise may increase butyrate-producing bacteria species [25,28]. Matsumoto, et al. [28] were the first to show that chronic voluntary physical exercise in animals is able to change SCFA production (n-butyrate) in the cecum with modifications in butyrate-producing bacteria species. In addition, this study reported alteration in the cecal microbiota profile after exercise. These authors explain that part of the beneficial effects of exercise related to microbiota and subsequent variations in intestinal health may be related to changes in the SCFA profile, especially for butyrate concentrations [28]. This shift in butyrate bacteria production in exercise group was also shown by Evans, et al. [25].
The influence of physical exercise on the composition of the microbial environment has been linked to a decreased pH in the gut from SCFA production. Specifically butyrate promotes cell differentiation and cell cycle arrest, inhibits the enzyme histone deacetylase, and decreases the transformation of primary to secondary bile acids promoting colonic acidification [36]. Changes in intestinal luminal pH may modify the environment in such way that it becomes more favorable for the proliferation of some bacterial species [37].
Studies also have shown that butyrate may induce mucin synthesis [38], and improve gut integrity by increasing tight junction assembly [34,39,40]. Mucins are the protective layer consisting of glycoproteins that help forming the mucosal barrier lining of gastrointestinal tract. This mucin layer has been recognized to play an important role for the interaction with gut microbiota, and may serve as a substrate for intestinal bacteria, as Akkermansia muciniphila, and may alter the microbial community composition [41].
Butyrate production in the large intestine is associated with production of Heat shock protein 70 (Hsp70). Hsp70 maintains the functional and structural properties of intestinal epithelial cells in response to intense exercise [42]. Since physical exercise and butyrate stimulate epithelial cell Hsp70 production, this may provide structural and functional stability to intestinal epithelial cells undergoing unfavorable conditions [25] ( Figure 1).
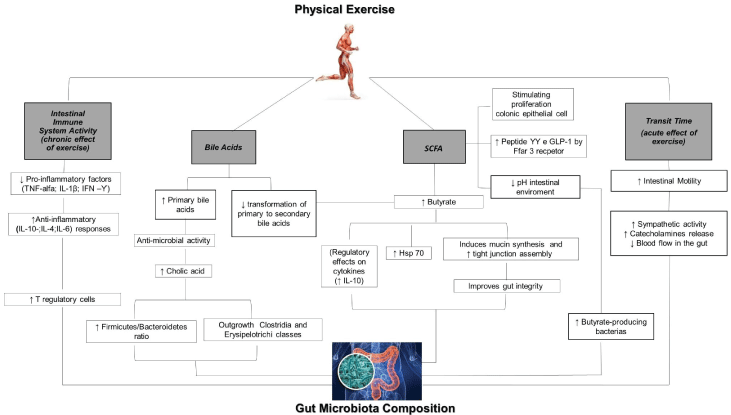 Figure 1: Schematic view representing the possible ways by which physical exercise may impact the gut microbiota. This includes Short-Chain Fatty Acid (SCFA) production by the microbiota, and bile acid production, as well as related alteration of the luminal pH. Exercise may also impact gut transit time and intestinal immune response, which in turn may modify the microbiota composition and metabolic activity. View Figure 1
Figure 1: Schematic view representing the possible ways by which physical exercise may impact the gut microbiota. This includes Short-Chain Fatty Acid (SCFA) production by the microbiota, and bile acid production, as well as related alteration of the luminal pH. Exercise may also impact gut transit time and intestinal immune response, which in turn may modify the microbiota composition and metabolic activity. View Figure 1
Physical activity has been reported to increase excretion of primary bile acids in the gastrointestinal tract. Since butyrate (that has been reported to be increased by physical activity) diminishes the conversion of bile acids into secondary bile acids, physical activity may consequently favor the rising of primary bile acids concentrations in the intestinal luminal content [23].
The primary bile acids have established anti-microbial activity. In agreement with this hypothesis, Islam, et al. [43] demonstrated that cholic acid induced substantial changes in the cecal microbiome composition by stimulating the growth of Firmicutes at the expense of Bacteroidetes; and provoked outgrowth of several bacteria in the Clostridia and Erysipelotrichi classes [44]. Antimicrobial activity of the bile acids may elicit selective pressure on the bacterial communities in exercised mice, leading to a shift of the gut microbiota composition [11].
Exercise and diet are considered as possible factors capable of modulating the intestinal balance between the hosts by independent manners. Exercise has been shown to improve the diversity of bacterial species and richness under different nutritional strategies thus allowing for instance to reduce the negative effects of high fat diet. The modification in short chain fatty acids production and alteration in intestinal pH appear to be the main forms by which exercise may affect gut microbiota composition.
It is important to note that, the studies performed up to now, used solely the voluntary exercise as model. The influence of specific features related to exercise training, such as volume, intensity, types of exercise (aerobic or anaerobic or combination) may impact gut microbiota in different ways. Likewise, changes in the diet and/or different pathological conditions in the experimental design raise some difficulties in evaluating only the exercise effect on the gut microbiota composition and metabolic activity, as well as in comparing the results obtained in different studies and this may hinder our understanding.
Further experiments, including molecular biology studies, are obviously required in order to delineate the precise mechanisms by which exercise impacts the intestinal microbiota. Studies involving human volunteers are also necessary to better elucidate the exercise-microbiota relationships and involved mechanisms.
This represents an important research area given the evident impact of physical exercise on gut microbiota composition and possible benefit on gut health.
The authors thanks CAPES/PROEX that support this publication.