International Journal of Sports and Exercise Medicine
Vacuum Sealing Drainage for Treating Early Infection after Total Hip Arthroplasty
Tao Liu, Fujiang Cao, Yunqiang Xu and Shiqing Feng*
Department of Orthopaedics Surgery, Tianjin Medical University General Hospital, China
*Corresponding author: Shiqing Feng, Department of Orthopaedics Surgery, Tianjin Medical University General Hospital,154 Anshan Road, Heping District, Tianjin, 300052, China, E-mail: fengsq9@126.com; fengsq9@gmail.com
Int J Sports Exerc Med, IJSEM-3-051, (Volume 3, Issue 1), Research Article; ISSN: 2469-5718
Received: July 08, 2016 | Accepted: February 10, 2017 | Published: February 14, 2017
Citation: Liu T, Cao F, Xu Y, Feng S (2017) Vacuum Sealing Drainage for Treating Early Infection after Total Hip Arthroplasty. Int J Sports Exerc Med 3:051. 10.23937/2469-5718/1510051
Copyright: © 2017 Liu T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Surgical site infections (SSIs) can have a profound impact on patients as they often require hospital readmission, additional surgical interventions, lengthy intravenous antibiotic administration, and delayed rehabilitation. Negative pressure wound therapy (NPWT) exposes the wound site to negative pressure, resulting in the improvement of blood supply, removal of excess fluid, and stimulation of cellular proliferation of granulation tissue. In this study, we investigated the clinical efficacy of the vacuum sealing drainage technique (VSD) for treating early infection after Total Hip Arthroplasty (THA).
Method: We reviewed the database of all patients treated at our center to identify those patients with infection after THA from Jan 2008 to Jan 2012. A total 1245 patients were performed THA, and 16 patients occurred infection. There were 6 male and 10 female. All the 16 patients went through a surgery probing the wound and thorough debridement was performed to remove the infected tissue and pus and implanted an VSD.
Results: Healing was successful for 16 patients (100%). All 16 patients occurred infection in three months after THA. The infection rate was 1.29%. All 16 patients occurred sinus and intra-articular infection. VSD was performed two times in 11 patients, and three times in 5 patients. After VSD and antibiotics treatment with mean 36-month follow-up, the patients had no evidence of infection and no infection recurred.
Conclusion: In this study, we found that VSD can control infection ideally, and the cure rate was 100%. We suggested that VSD can be a perfect treatment programs for patients with infection after HTA in acute phase.
Keywords
VSD, Infection, THA
Introduction
Total hip arthroplasty (THA) is indicated for treating chronic refractory joint pain and some types of proximal femoral fractures. It is one of the most successful operations in orthopaedic surgery with outstanding long-term outcomes in terms of reduction of pain and restoration of function [1]. However, increasing incidence of revision due to infection after primary total hip arthroplasty (THA) has been observed in different countries during the last decade [2,3]. Infection of a total hip prosthesis is a potentially catastrophic event associated with an increased risk of postoperative complications and with significant impairment in function and quality of life [4]. Moreover, the cost of treating patients with infected total hip prostheses is high [5]. Therefore, strategies for preventing and treating infections are important.
Infection following THA can be classified based on the timing of infection: early is defined as occurring within 1 month of prosthesis implantation, delayed occurring between 1 month and 1 year, and late infection occurring more than 1 year after implantation [6]. For infection after THA, there are numerous reports of successful results of infection control. However, most of them are related to stage revision and long-term antibiotic suppression therapy [7,8]. The economic burden, physical and psychological damage were enormous for patients. Therefore, early diagnosis and treatment are important for avoiding stage revision.
Since its introduction into clinical care over a decade ago, negative pressure wound therapy (NPWT) has become a prevalent treatment modality used to promote the healing of acute wounds, chronic wounds, and skin grafts. The exact mechanism of NPWT wound healing is still an active area of research; nevertheless, evidence suggests it is accomplished through increasing blood circulation by angiogenesis, removing edema, increasing granulation tissue formation, and decreasing bacteria counts, all while increasing patient comfort. In this study, we reported the vacuum sealing drainage technique (VSD) for treating early infection after THA. Vacuum sealing drainage is already in widespread use for wound restoration in orthopedic departments. It facilitates deferred restoration by providing optimal protection for fresh wounds. Especially for infected tissue, early radical debridement and provisional VSD cover can elicit inflammatory adequately, improve local microcirculation and promote wound recovery. Here, we posed the following questions: (1) Whether the VSD is useful for controlling early infection after THA? (2) Whether prosthesis needed be removed? (3) Whether the patients need a stage revision?
Materials and Methods
Patients
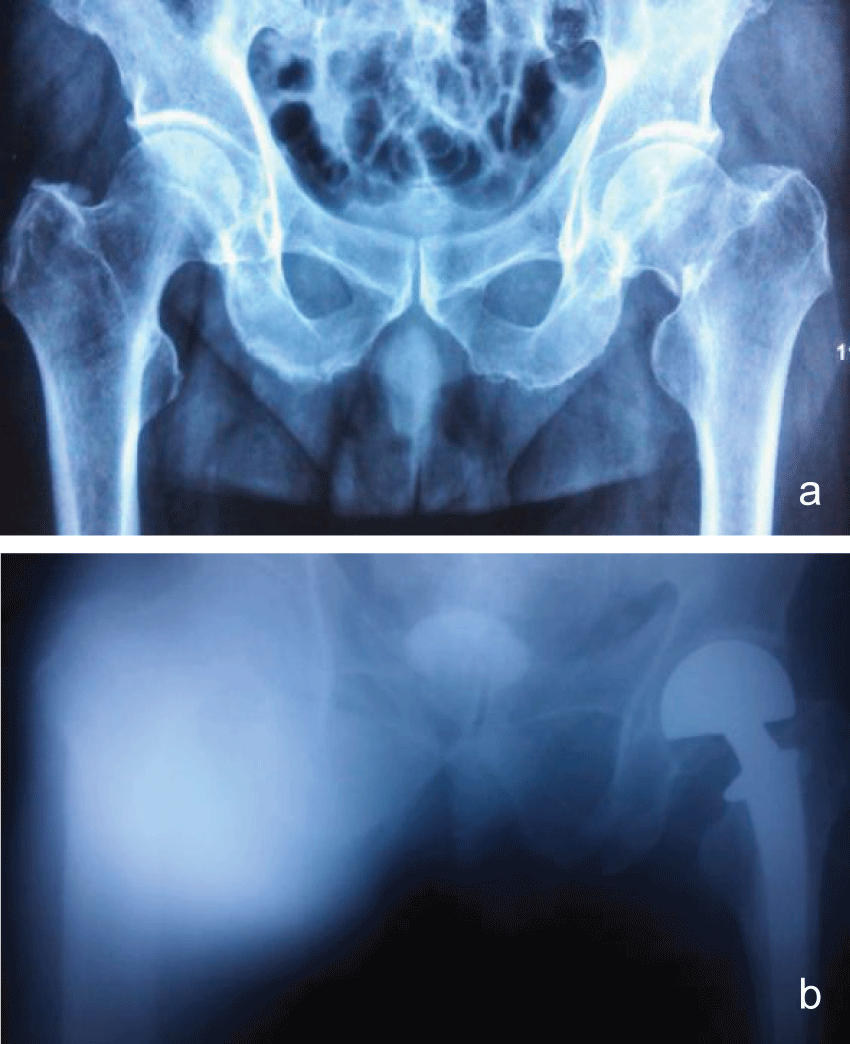
We reviewed the database of all patients treated at our center to identify those patients with infection after THA from Jan 2008 to Jan 2012. A total 1245 patients were performed THA, and 16 patients occurred infection. There were 6 male and 10 female. The mean age at the time of initial surgical treatment was 65 (range, 41-86) years, and the mean follow up was 36 (range, 18-48) months. The underlying diagnosis leading to the primary THA was idiopathic aseptic femoral head necrosis (IAFHN) in five patients, acute femoral neck fracture in eleven patients (Table 1).
![]()
Table 1: Patients demographic characteristics.
View Table 1
Diagnosis
A diagnosis of infection was made when there was drainage of pus, positive culture of aspirated fluid and/or tissue, or histological evidence of infection [9]. When the culture was negative, final diagnosis was made by the surgeons' clinical decision based on clinical symptoms, signs, and laboratory data. Our cut-offs for abnormal laboratory findings were: erythrocyte sedimentation rate (ESR) greater than 25 mm/hr, C-reactive protein (CRP) greater than 8 mg/L, and joint aspirate white blood cell count (WBC) greater than 2000/μL with polymorphonuclear differential count greater than 65% [10,11].
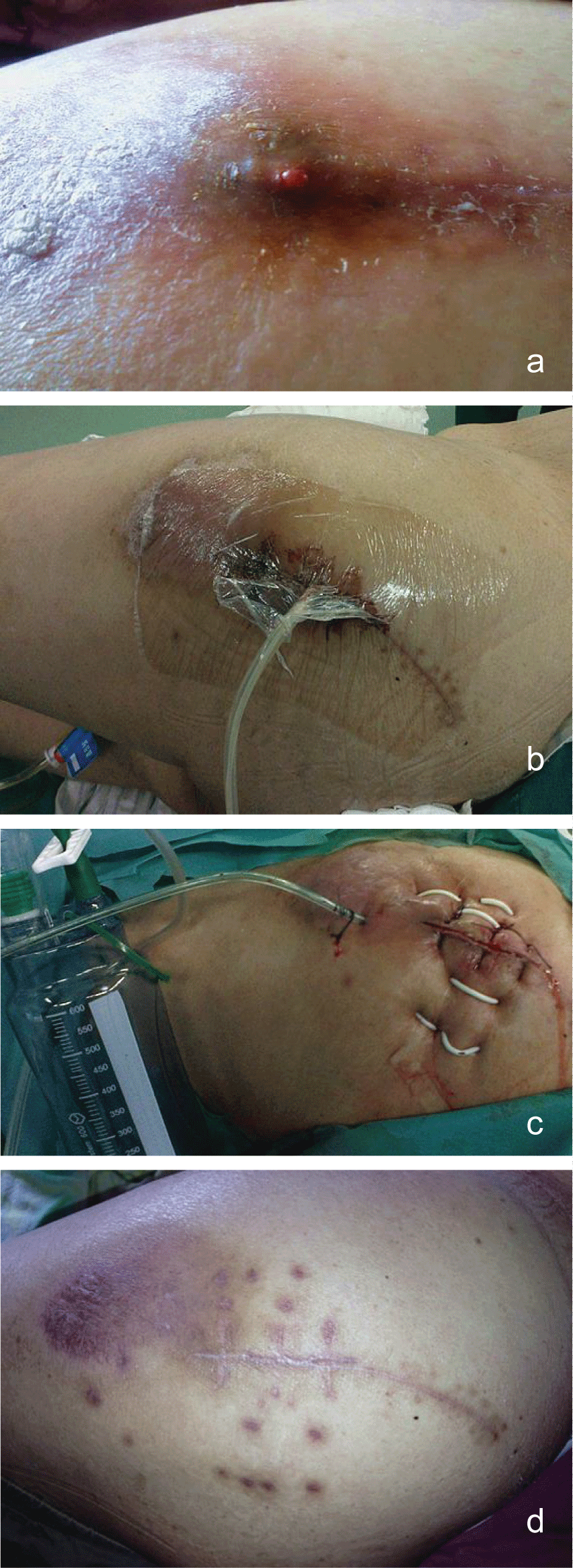
Physical examination of the inflamed limb revealed a discolored and oedematous femoral region, increased temperature when compared with the contralateral limb and pus oozing from a recent puncture site at the affected site. The pain in the affected limb during physical examination was out of proportion to the external physical appearance of atypical cellulitis. Based on the above, the decision to proceed to the operating room was taken and the initial aggressive and wide surgical debridement led to the evacuation of an extended pus collection in the superior anterior femoral muscular compartment and the removal of necrotic muscle tissue (myonecrosis affected the middle head of the quadriceps muscle mostly). The evacuated necrotic tissue area was in direct contact with the femoral vessels. Tissue samples and cultures were taken and sent to the microbiology department. Vacuum sealing drainage device was applied to the traumatic site (Figure 1a and Figure 1b).The patient was subsequently admitted to the surgical ward to support the basic life systems and monitor the response to therapy.
.
Figure 1: a) Infection after THA; b) VSD was performed; c) VSD was removed and incision was sutured with drainage; d: Infection was recovery.
View Figure 1
VSD device
VSD dressing was produced by Weisidi Medical Technology Co., Ltd located in Wuhan, China (Specifications: 150 mm × 100 mm × 9 mm; 150 mm × 50 mm × 9 mm), which interpolated with porous silicone drainage tube. Biological semipermeable membrane was produced by Smith & Nephew Medical Technology Co., Ltd. Vacuum device was keep at 0.02-0.04 kPa in sickroom. All of the abovementioned materials were packaged in an aseptic manner.
Operative technique
Based on the physical examination, the decision to proceed to the operating room was taken and the initial aggressive and wide surgical debridement led to the evacuation of an extended pus collection in the superior anterior femoral muscular compartment and the removal of necrotic muscle tissue (myonecrosis affected the middle head of the quadriceps muscle mostly). The evacuated necrotic tissue area was in direct contact with the femoral vessels. Tissue samples and cultures were taken and sent to the microbiology department. Vacuum sealing drainage device was applied to the traumatic site (Figure 1b).The patient was subsequently admitted to the surgical ward to support the basic life systems and monitor the response to therapy.
Under general anesthesia with endotracheal intubation, the patients were placed lateral position. After routine disinfection, the skin was incised along the distal end of original incision, which included the ulcerated wounds. Probing the wound and thorough debridement were performed to remove the infected tissue and pus. During the procedure, several samples were sent for culture and sensitivity tests, and histological evaluation. Then the wound was washed repeatedly with large quantities of saline solution and hydrogen peroxide. After initial extensive debridement wounds were filled with a Poly (vinyl alcohol) shrink formaldehyde bubble using porous silicone drainage tube according to the wound size. The Poly (vinyl alcohol) shrink formaldehyde bubble was stuck to the healthy skin, and the wound was then closed with Poly amino acid ethylester films (Figure1a and Figure 1b).
Postoperative management
Vacuum device was keep at 0.02-0.04 kPa in sickroom and observed daily. Saline should be used to rinse if there was a blockage in drainage tube. Initial empiric antibiotic coverage included high dose vancomycin (1g Q12h, i.v.). It was later adjusted to cover the cultured microorganisms. All patients were accepted iv antibiotics (vancomycin) treatment until the VSD removed, while they were at hospital, and the all patients took rifampicin capsules for half years since they were discharged from hospital. The choice of antibiotics was up to the results of culture of aspirated fluid or blood and drug sensitiveness test. A second VSD dressing was decided according to the soft tissue situation and sinus recovery. The VSD was changed every week until the culture of aspirated fluid and tissue both were negative, which would be done three times. We closed the wound after the VSD was removed. And a drainage would be left for 3 days (Figure 1c, Figure 1d, Figure 2a and Figure 2b).
Results
All 16 patients occurred infection in three months after THA. The infection rate was 1.29%. All 16 patients occurred sinus and intra-articular infection. VSD was performed two times in 11 patients, and three times in 5 patients. After VSD and antibiotics treatment with mean 36-month follow-up, the patients had no evidence of infection and no infection recurred (Table 2).
![]()
Table 2: Patients clinical character characteristics.
View Table 2
Discussion
VSD is a kind of surgical technological innovation which applied to treat trauma, infection and osteomyelitis and so on. It is a new method of deep drainage which can remove the secretions and necrotic tissue of lacunar or wound. Especially for infection tissue, pus can be drained continuously and totally. And the tissue can be kept in a healthy microenvironment which promotes infection healing.
Surgical site infection is a major concern for surgeons in all specialties [12]. Infection after THA is rare but potentially devastating. While the risk of infection after primary THA has been reduced to approximately 1% in most cases [13-15], there remain a substantial percentage of patients who became reinfected after two-stage reimplantation. These reinfections can be extremely difficult to eradicate, costing too much per revision. However, two-stage arthroplasty is still considered the standard therapeutic approach [16-18] for infection after THA. In general, retention treatment reportedly has lower success rates than staged revision arthroplasty [19,20]. However, there was no study had reported using VSD for controlling the infection after THA to avoid stage revision. In this study, we got an exciting result that VSD can control deep infection in acute phase after THA. What's more, the prostheses can be retained, and a stage revision was not required. This technique alleviated patient's sufferings and economic burden.
In Choi HR's study [11], they reported that in the retention and removal groups, infection control rates were 50% and 78% after initial treatment, and 68% and 82% at latest follow-up, respectively. Most patients (28/38, 78%) received retention treatment in the acute phase and all patients received removal treatment in the chronic phase (n = 55). In our study, all the patients suffered infection in acute phase, and the infection control rates were 100% using retention treatment with VSD. We analyzed that the infection was limited and prostheses was stable in acute phase, so that VSD can control infection ideally and can be a first choice for infection after HTA in acute phase.
Conclusion
Infection is a kind of common and serious complication after total hip arthroplasty. Stage revisions always bring sufferings and economic burden to patients. In this study, we found that VSD can control infection ideally, and the cure rate was 100%. We suggested that VSD can be a perfect treatment programs for patients with infection after HTA in acute phase.
Conflict of Interest
The authors declare no conflict of interest.
Authors' Contributions
The design of the study was done by SF. TL and YX prepared the manuscript and assisted in the study processes. FC assisted in the data collections. All authors read and approved the final manuscript.
Acknowledgements
We thank Dr. Shiqing Feng for his support in obtaining the approval of the ethics committee in this study.
This work was funded by National Science Foundation for Young Scholars of China (Grant 81401784). No benefits in any form have been or will be received from any commercial party related directly and indirectly to the subject of this manuscript.
References
-
Laupacis A, Bourne R, Rorabeck C, Feeny D, Wong C, et al. (1993) The effect of elective total hip replacement on health-related quality of life. J Bone Joint Surg Am 75: 1619-1626.
-
Dale H, Hallan G, Hallan G, Espehaug B, Havelin LI, et al. (2009) Increasing risk of revision due to deep infection after hip arthroplasty. Acta Orthop 80: 639-645.
-
Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, et al. (2008) Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty 23: 984-991.
-
Biring GS, Kostamp T, Garbuz DS, Masri BA, Duncan CP (2009) Two-stage revision arthroplasty of the hip for infection using an interim articulated Prostalac hip spacer: a 10- to 15-year follow-up study. J Bone Joint Surg Br 91: 1431-1437.
-
Klouche S, Sariali E, Mamoudy P (2010) Total hip arthroplasty revision due to infection: a cost analysis approach. Orthop Traumatol Surg Res 96: 124-132.
-
Wu F, Marriage NA, Ismaeel A, Masterson E (2011) Infection of a total hip arthroplasty with actinomyces israelii: Report of a case. N Am J Med Sci 3: 247-248.
-
Crockarell JR, Hanssen AD, Osmon DR, Morrey BF (1998) Treatment of infection with débridement and retention of the components following hip arthroplasty. J Bone Joint Surg Am 80: 1306-1313.
-
Waagsbo B, Sundoy A, Martinsen TM, Nymo LS (2009) Treatment results with debridement and retention of infected hip prostheses. Scand J Infect Dis 41: 563-568.
-
Estes CS, Beauchamp CP, Clarke HD, Spangehl MJ (2010) A two-stage retention debridement protocol for acute periprosthetic joint infections. Clin Orthop Relat Res 468: 2029-2038.
-
Trampuz A, Hanssen AD, Osmon DR, Mandrekar J, Steckelberg JM, et al. (2004) Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am J Med 117: 556-562.
-
Choi HR, von Knoch F, Kandil AO, Zurakowski D, Moore S, et al. (2012) Retention treatment after periprosthetic total hip arthroplasty infection. Int Orthop 36: 723-729.
-
Wenzel RP (2010) Minimizing surgical-site infections. N Engl J Med 362: 75-77.
-
Whittaker JP, Warren RE, Jones RS, Gregson PA (2009) Is prolonged systemic antibiotic treatment essential in two-stage revision hip replacement for chronic Gram-positive infection? J Bone Joint Surg Br 91: 44-51.
-
Cierny G, DiPasquale D (2002) Periprosthetic total joint infections: staging, treatment, and outcomes. Clin Orthop Relat Res: 23-28.
-
Johnson AJ, Zywiel MG, Jones LC, Delanois RE, Stroh DA, et al. (2013) Reduced re-infection rates with postoperative oral antibiotics after two-stage revision hip arthroplasty. BMC Musculoskelet Disord 14: 123.
-
Sanchez-Sotelo J, Berry DJ, Hanssen AD, Cabanela ME (2009) Midterm to long-term followup of staged reimplantation for infected hip arthroplasty. Clin Orthop Relat Res 467: 219-224.
-
Schmalzried TP (2006) The infected hip: telltale signs and treatment options. J Arthroplasty 21: 97-100.
-
Toms AD, Davidson D, Masri BA, Duncan CP (2006) The management of peri-prosthetic infection in total joint arthroplasty. J Bone Joint Surg Br 88: 149-155.
-
Hanssen AD, Spangehl MJ (2004) Treatment of the infected hip replacement. Clin Orthop Relat Res 63-71.
-
Zimmerli W, Trampuz A, Ochsner PE (2004) Prosthetic-joint infections. N Engl J Med 351: 1645-1654.