International Journal of Sports and Exercise Medicine
Controlled Trial on the Effects of Creatine Supplementation on Muscle Mass and Function among Older Women Subjected to Resistance Training
María José Prieto Kinesiologist1, Marcela Catalán Kinesiologist1, Gonzalo Ayala Kinesiologist1, Nicolás Gajardo Kinesiologist1, Gladys Barrera Registered Nurse2, Sandra Hirsch2, María Pia de la Maza2 and Daniel Bunout2*
1Department of Kinesiology, Universidad Metropolitana de Ciencias de la Educación, Chile
2Institute of Nutrition and Food Technology, University of Chile, Chile
*Corresponding author: Daniel Bunout MD, Institute of Nutrition and Food Technology, INTA University of Chile, PO Box 138-11, Santiago, Chile, Tel: +56-(2)-29781485, Fax: +56-(2)-22214030, E-mail: dbunout@inta.uchile.cl
Int J Sports Exerc Med, IJSEM-2-048, (Volume 2, Issue 5), Original Article; ISSN: 2469-5718
Received: June 29, 2016 | Accepted: Novomber 25, 2016 | Published: December 01, 2016
Citation: Kinesiologist MJP, Kinesiologist MC, Kinesiologist GA, Kinesiologist NG, Nurse GBR, et al. (2016) Controlled Trial on the Effects of Creatine Supplementation on Muscle Mass and Function among Older Women Subjected to Resistance Training. Int J Sports Exerc Med 2:048. 10.23937/2469-5718/1510048
Copyright: © 2016 Kinesiologist MJP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: The effects of creatine supplementation in older people are dubious.
Aim: To assess the effects of creatine supplementation in older women subjected to resistance training.
Material and Methods: Older women aged between 60 and 75 years without chronic debilitating diseases, living in the community were invited to participate. All were subjected to supervised resistance training using elastic bands, three times per week during 12 weeks and were randomized to receive, in a double blind fashion, 5 g/day of creatine monohydrate or maltodextrin, during the same period. At baseline and the end of the intervention period, blood pressure was measured, body composition was evaluated by double energy X-ray absorptiometry (DEXA), quadriceps torque was assessed in a quadriceps table, timed up and go (TUG) and the 12 minutes' walk tests were carried out. Compliance with the supplement and training sessions was evaluated periodically recording the attendance to training sessions, evaluating subjective effort during exercise sessions using the OMNI-Resistance Exercise Scale and counting the leftover sachets of creatine or placebo.
Results: Fifty participants were randomized and 39 (17 in the placebo and 22 in the creatine group), completed the intervention. Among the latter, compliance with training and the supplement was over 80%. After the intervention period, total and appendicular fat free mass, right quadriceps torque and twelve minutes test increased significantly in all participants. Significant reductions in diastolic blood pressure and hip circumference were also observed. No significant differences were observed in the change of these parameters between participants receiving the active supplement or placebo.
Conclusions: Creatine supplementation had no effect on muscle mass or function in these women.
Keywords
Nutritional supplement, Exercise, Older adults
Introduction
Sarcopenia or the loss of muscle mass and function with age is associated with a higher risk of dependence, worst prognosis during hospitalizations and lower survival. Exercise training is one of the few effective interventions for sarcopenia. However, the positive effects of exercise training in older people are hampered by the lack of compliance with training sessions and their short duration. The experience in community-based training programs, lasting one to three years is that the Attendance is approximately 50% and that the attrition rate at one year is approximately 30% [1,2]. In addition, the positive results of training wane in approximately one month after stopping the intervention [3]. Therefore, the exploration of other therapeutic options for sarcopenia is worthwhile. One of these is the use of ergogenic compounds such as creatine. The use of whey protein or leucine supplementation also increases muscle mass, when combined with vitamin D [4]. Beta-hydroxy-beta-methylbutyrate (HMB) supplementation could also exert a positive effect but good quality evidence is lacking [5].
In young people, a meta-analysis showed that creatine supplementation has a minimal potentiating effect on resistance training, increasing muscle mass and strength [6]. However, the effects of creatine supplementation in people aged 50 years or more are dubious. While some studies show improvements in muscle mass, strength and functional tests [7-10], other studies fail to show such benefit [11-14].
Thus, the aim of this study was to evaluate the effects on fat free mass and muscle strength of a creatine monohydrate supplementation during 12 weeks, in women aged 60-years-old or older, subjected to resistance training, in a double blind trial.
Methods
Fifty women of middle and low socioeconomic level defined according to their monthly income and the commune where they reside, living in the community and aged between 60 and 75 years, were invited to participate in the study. They were recruited in a period of three months immediately before starting the intervention. Exclusion criteria were smoking ever or excessive consumption of alcohol (defined as more than 15 g of alcohol per day), use of medications that could limit exercise capacity such as steroids, muscle relaxants or statins in high doses, having a clinically evident hepatic, cardiac or renal disease, having a severe osteoarticular disease, being engaged in an active exercise training program in the last six months and having a cognitive dysfunction or physical disability.
At baseline, the following assessments were carried out:
1. A complete medical history including use of medications, previous diseases and surgical procedures.
2. Evaluation of usual physical activity using the International Physical Activity Questionnaire (IPAQ) [15].
3. Mini nutritional assessment [16]. The 2013 version available in internet was used (www.mna-elderly.com/forms/MNA_spanish.pdf) and the total score was calculated.
4. Measurement of weight, height, waist and hip circumference.
5. Measurement of body composition by double energy X-ray absorptiometry in a General Electric iDEXA equipment. The variability of the measurement is less than 3% [17].
6. Measurement of rectus femoris cross sectional height using a General Electric LogiQ ultra sonographer with the participant in supine position and the thigh muscles relaxed. Measurements were made using a GE 12L-RS 14.2 × 47 mm linear array ultrasound transducer probe. The mid-point between the superior anterior iliac spine and the superior border to the patella was set as the measurement point.
7. Measurement of quadriceps isometric strength as previously described [18]. A quadriceps table equipped with an isometric force transducer, connected to a computer to register the maximal strength achieved in one repetition was used. Three consecutive measurements were done and the best value was registered. Strength was expressed in Newtons (N).
8. Measurement of hand grip strength using a hand grip dynamometer T-18 (Country Technology, Inc.) with a precision of 0.1 kg, in both hands. The handle of the dynamometer was regulated to accommodate the hand size of participants, who were requested to squeeze the dynamometer as hard as they could, while standing. Three measurements were made and the higher value was recorded.
9. Measurement of 12 minutes walking capacity [19].
10. Measurement of Timed Up and Go. (TUG) [20]. Three attempts were made and the lower time required was recorded in seconds (s).
Intervention and Randomization
After performing the baseline assessment, participants were randomized in one of two groups, balancing by age and nutritional status, using a double blind design. A specially designed software randomly allocated participants to each group. An iteration of the software randomly allocated the participants to each group repeatedly until no significant differences in age or body mass index between groups were detected.
a. The active group received creatine 5 g/day in one dose as a powder packed in a sachet to be dissolved in water.
b. The control group received a placebo (maltodextrin) of similar aspect and taste as creatine, packed in an identical sachet.
The active prescription and the placebo were identified by a unique numeric code. The codes were known by an external professional, not involved in the research. Every two weeks, a new supply of creatine or placebo was delivered and the participants were requested to return the unused sachets.
All participants were incorporated to a supervised exercise training program, which lasted 12 weeks, with three sessions of 45 minutes per week. All training sessions were carried out at the Institute of Nutrition and Food Technology facilities, under the supervision of four of the authors (MJP, MC, GA, NG). Each exercise session consisted of:
a. A warm-up period of 15 minutes, consisting in brisk walking.
b. A resistance training program, based on progressive overload using Thera-Band® elastic bands. Chair stands, modified squats, step-ups in a stair and arm pull-ups were performed. The training protocol started performing one set of each exercise at 60-80% of their 1 Repetition Maximum (RM) and progressed thereafter. For each muscle group, three sets of repetitions were carried out. All exercises were calibrated to be of high intensity according to the OMNI-Resistance Exercise Scale values during the training period [21]. Intensity was progressively increased by 10%, every two weeks until the sixth week. Afterwards, it remained at 80% of a RM.
c. An elongation exercise period lasting 10 minutes that included elongation in Thera-Band® mats and exercise balls.
The attendance of participants to each session, was recorded. All adverse events reported by participants, were also recorded following good clinical practice guidelines [22].
At the end of 12 weeks of intervention, all the assessments done at baseline, except the IPAQ questionnaire, were repeated within one week after the last training session.
The main outcome was an increase in fat free mass. The secondary outcomes were increases in quadriceps torque or walking capacity with creatine supplementation, compared to placebo.
The study was approved by the Institute of Nutrition and Food Technology of the University of Chile ethics committee and all participants signed a written informed consent to participate in the study
Statistics
All analyzes were done blinded to the allocation of participants (placebo or creatine group), using Stata 12 for Windows (Statacorp, College Station, Texas 77845, USA). The compliance with the creatine or placebo supplement was determined counting the number of unused sachets returned by participants. The compliance with exercise sessions was determined calculating the percentage of programmed training sessions that each participant attended. Normality of variable distribution was determined using the Shapiro-Wilk test. Normally distributed variables are expressed as mean ± standard deviation, otherwise as median (range). Differences between normally distributed variables were analyzed using Student's t-test. Changes in parameters were assessed using a repeated measures ANOVA to find possible significant effects of the intervention and possible interactions between groups. A probability of 5% or less to reject the null hypothesis, was considered significant. An intention to treat analysis was performed
The number of participants was calculated to provide a power of 0.8 to detect a 17% difference in appendicular fat free mass and a 15% difference in quadriceps torque between groups. These cutoff points were based on previous reports on the effects of creatine supplementation.
Results
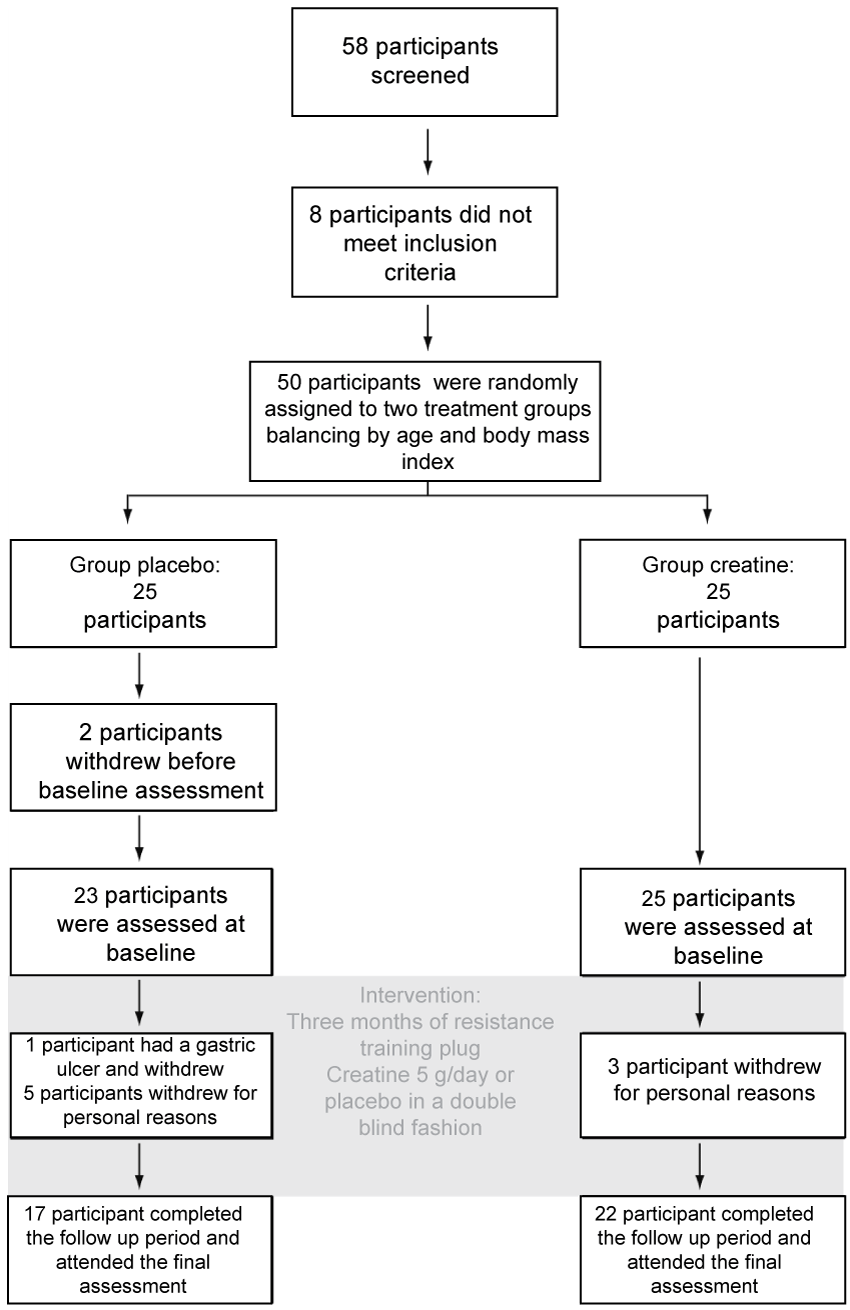
Participant flow is shown in figure 1. Fifty participants were randomized and 39 completed the three months intervention period (17 in group placebo and 22 in group creatine). Among participants dropping out, seven decided not to continue after being randomized for personal reasons, three abandoned due to prolonged holidays and one lost interest in the protocol. The baseline features of all participants randomized is shown in table 1. The medical background and medications used by participants is shown in table 2. Among those who completed the intervention period, the compliance with the supplement was 88.9 (67-100) and 91.1 (51-99) % among participants in groups placebo and creatine, respectively. The figures for attendance to training sessions were 85 (75-85) and 85 (77-85) % among participants in groups placebo and creatine, respectively. During the intervention period, adverse events were reported by 10 and 11 participants of group's placebo and creatine, respectively. Five and nine of these events were qualified as moderate in group's placebo and creatine respectively. The rest of the events were qualified as mild. Five events in each group were considered as related or possibly related with the exercise intervention (Table 3).
![]()
Table 1: Baseline features of all patients evaluated at baseline (mean ± standard deviation).
View Table 1
![]()
Table 2: Number of participants with concomitant medical conditions and medication use.
View Table 2
The evolution of parameters after the intervention is shown in table 4. A significant increase in right quadriceps torque, twelve minutes' walk, total and appendicular fat free mass and waist circumference, was observed in both groups with no differences between treatment groups. Significant reductions in diastolic blood pressure and hip circumference were also observed with no differences between groups. These results do not change if only participants with a compliance greater than 80% with training or the supplement, are analyzed.
![]()
Table 3: Details of adverse events reported by participant.
View Table 3
![]()
Table 4: Changes in clinical and laboratory parameters during the intervention. Results are expressed as mean ± standard deviation.
View Table 4
Discussion
In this study, performed in healthy elderly women, resistance training increased muscle mass and strength, but creatine supplementation had no additional effect on these parameters.
As outcomes, we measured body composition, muscle strength and overall functional tests. These are commonly used in clinical trials with resistance exercise and nutritional supplements. DEXA is the current gold standard to measure fat free body mass. It has the limitation of being influenced by the hydration status of individuals [23]. This problem was not an issue among these women who did not have severe cardiac or renal diseases that could cause hydration disturbances. Hand grip strength and quadriceps torque were used to assess muscle function. Mobility was assessed with the Timed Up and Go, a commonly use functional test in older people. Twelve minutes walking is an endurance test, which could also improve along with lean body mass and strength [24,25].
We observed that blood pressure decreased after the intervention. The blood pressure reduction effects of exercise are well known and do not deserve further discussion [26] and are probably related to the benefits of exercise on cardiovascular health and survival [27].
The lack of effects of creatine supplementation on muscle mass or strength is in accordance with many, but not all, previous reports [13]. It may be due to a real lack of effect of the supplement or to design limitations of the present intervention. This last possibility is unlikely since we tried to avoid most confounding factors in the protocol design. We chose participants of one gender since it influences body composition and response to training [25]. We excluded participants with diseases such as clinically evident hepatic, cardiac or renal failure that could influence the results and most importantly, we used a strict double blind design, even during data analysis to avoid any bias caused by preconceptions. Our results are in contradiction with other reports showing a positive effect of creatine supplementation [7,28]. The discrepancies with our study may be due to several factors such as the age and gender of participants and timing of creatine supplementation. The age of participants in previous studies was lower and in general, both genders were studied simultaneously. If creatine is provided immediately before the training session, the increase in muscle blood flow during exercise should enhance creatine uptake by skeletal muscle [29]. However, creatine levels remain elevated in the blood for approximately 6 hours after ingestion, refuting the argument that a strict dosing timing in relation to exercise is necessary [28]. Therefore, albeit timing of creatine ingestion could be important, it does not seem to be a limiting factor in its possible beneficial effects, especially considering the steady increases in plasma, red blood cell and muscle creatine concentrations observed after repetitive dosing of the supplement [30,31]. Moreover, the dose used in the present study allows to maintain, in the long term, steadily increased muscle concentrations [32]. However, we used a fixed dose of creatinine for all patients and other authors calculate the dose per kg of body weight [11,14]. We cannot exclude this fact as a cause for not finding an effect of the supplement.
Conclusions
No effect of creatine supplementation was observed in this group of older women subjected to resistance training.
Funding
This work was supported by Fondecyt Grant # 1130284 and the Thera-Band Academy. The funders had no influence in the design and conduction of the trial or analysis of results.
Conflicts of Interest
The authors declare to have no conflict of interest.
Clinical Trials Registration
NCT02188849. Protocol can be accessed at www.clinicaltrials.com.
References
-
Bunout D, Barrera G, Leiva L, Gattas V, de la Maza MP, et al. (2006) Effects of vitamin D supplementation and exercise training on physical performance in Chilean vitamin D deficient elderly subjects. Exp Gerontol 41: 746-752.
-
Garmendia ML, Dangour AD, Albala C, Eguiguren P, Allen E, et al. (2013) Adherence to a physical activity intervention among older adults in a post-transitional middle income country: a quantitative and qualitative analysis. J Nutr Health Aging 17: 466-471.
-
Hakkinen K, Alen M, Kallinen M, Newton RU, Kraemer WJ (2000) Neuromuscular adaptation during prolonged strength training, detraining and re-strength-training in middle-aged and elderly people. Eur J Appl Physiol 83: 51-62.
-
Bauer JM, Verlaan S, Bautmans I, Brandt K, Donini LM, et al. (2015) Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 16: 740-747.
-
Alon T, Bagchi D, Preuss HG (2002) Supplementing with beta-hydroxy-beta-methylbutyrate (HMB) to build and maintain muscle mass: a review. Res Commun Mol Pathol Pharmacol 111: 139-151.
-
Nissen SL, Sharp RL (2003) Effect of dietary supplements on lean mass and strength gains with resistance exercise: a meta-analysis. J Appl Physiol (1985) 94: 651-659.
-
Bermon S, Venembre P, Sachet C, Valour S, Dolisi C (1998) Effects of creatine monohydrate ingestion in sedentary and weight-trained older adults. Acta Physiol Scand 164: 147-155.
-
Gotshalk LA, Volek JS, Staron RS, Denegar CR, Hagerman FC, et al. (2002) Creatine supplementation improves muscular performance in older men. Med Sci Sports Exerc 34: 537-543.
-
Brose A, Parise G, Tarnopolsky MA (2003) Creatine supplementation enhances isometric strength and body composition improvements following strength exercise training in older adults. J Gerontol A Biol Sci Med Sci 58: 11-9.
-
Aguiar AF, Januário RS, Junior RP, Gerage AM, Pina FL, et al. (2013) Long-term creatine supplementation improves muscular performance during resistance training in older women. Eur J Appl Physiol 113: 987-96.
-
Canete S, San Juan AF, Pérez M, Gómez-Gallego F, Lopez-Mojares LM, et al. (2006) Does creatine supplementation improve functional capacity in elderly women? J Strength Cond Res 20: 22-28.
-
Eijnde BO, Van Leemputte M, Goris M, Labarque V, Taes Y, et al. (2003) Effects of creatine supplementation and exercise training on fitness in men 55-75 yr old. J Appl Physiol (1985) 95: 818-828.
-
Bemben MG, Witten MS, Carter JM, Eliot KA, Knehans AW, et al. (2010) The effects of supplementation with creatine and protein on muscle strength following a traditional resistance training program in middle-aged and older men. J Nutr Health Aging 14: 155-159.
-
Candow DG, Zello GA, Ling B, Farthing JP, Chilibeck PD, et al. (2014) Comparison of creatine supplementation before versus after supervised resistance training in healthy older adults. Res Sports Med 22: 61-74.
-
Kim Y, Park I, Kang M (2013) Convergent validity of the international physical activity questionnaire (IPAQ): meta-analysis. Public Health Nutr 16: 440-452.
-
Schrader E, Baumgärtel C, Gueldenzoph H, Stehle P, Uter W, et al. (2014) Nutritional status according to mini nutritional assessment is related to functional status in geriatric patients--independent of health status. J Nutr Health Aging 18: 257-263.
-
Gajardo H, Barrera G (1998) [Quality control of bone densitometry: precision, reproducibility, and clinical application]. Rev Med Chil 126: 56-62.
-
Bunout D, Barrera G, Avendaño M, de la Maza P, Gattas V, et al. (2005) Results of a community-based weight-bearing resistance training programme for healthy Chilean elderly subjects. Age Ageing 34: 80-83.
-
McGavin CR, Gupta SP, McHardy GJ (1976) Twelve-minute walking test for assessing disability in chronic bronchitis. Br Med J 1: 822-823.
-
Bohannon RW (2006) Reference values for the timed up and go test: a descriptive meta-analysis. J Geriatr Phys Ther 29: 64-68.
-
Colado JC, Garcia-Masso X, Triplett TN, Flandez J, Borreani S, et al. (2012) Concurrent validation of the OMNI-resistance exercise scale of perceived exertion with Thera-band resistance bands. J Strength Cond Res 26: 3018-3024.
-
(2016) Good Clinical Practice: Consolidated Guidance.
-
Georgiou E, Virvidakis K, Douskas G, Lambrinoudaki I, Voudiklari S, et al. (1997) Body composition changes in chronic hemodialysis patients before and after hemodialysis as assessed by dual-energy x-ray absorptiometry. Metabolism 46: 1059-1062.
-
de Greef MH, Sprenger SR, Elzenga CT, Popkema DY, Bennekers JH, et al. (2005) Reliability and validity of a twelve-minute walking test for coronary heart disease patients. Percept Mot Skills 100: 567-575.
-
Bunout B, Barrera G, de la Maza P, Avendano M, Gattas V, et al. (2004) Effects of nutritional supplementation and resistance training on muscle strength in free living elders. Results of one year follow. J Nutr Health Aging 8: 68-75.
-
Cornelissen VA, Smart NA (2013) Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Assoc 2: e004473.
-
Manini TM, Everhart JE, Patel KV, Schoeller DA, Colbert LH, et al. (2006) Daily activity energy expenditure and mortality among older adults. JAMA 296: 171-179.
-
Candow DG, Vogt E, Johannsmeyer S, Forbes SC, Farthing JP (2015) Strategic creatine supplementation and resistance training in healthy older adults. Appl Physiol Nutr Metab 40: 689-694.
-
Schedel JM, Tanaka H, Kiyonaga A, Shindo M, Schutz Y (1999) Acute creatine ingestion in human: consequences on serum creatine and creatinine concentrations. Life Sci 65: 2463-2470.
-
Brault JJ, Towse TF, Slade JM, Meyer RA (2007) Parallel increases in phosphocreatine and total creatine in human vastus lateralis muscle during creatine supplementation. Int J Sport Nutr Exerc Metab 17: 624-634.
-
Watt KK, Garnham AP, Snow RJ (2004) Skeletal muscle total creatine content and creatine transporter gene expression in vegetarians prior to and following creatine supplementation. Int J Sport Nutr Exerc Metab 14: 517-531.
-
Preen D, Dawson B, Goodman C, Beilby J, Ching S (2003) Creatine supplementation: a comparison of loading and maintenance protocols on creatine uptake by human skeletal muscle. Int J Sport Nutr Exerc Metab 13: 97-111.