International Journal of Sports and Exercise Medicine
Correlations between Vitamin D Concentrations and Lipid Panels in Active Duty and Veteran Military Personnel
Ashlyn M. Hiserote1, Cristóbal S. Berry-Cabán2, Qiang Wu3 and Laurel M. Wentz1*
*Corresponding author: Laurel M. Wentz, PhD, RD, CSSD, LDN, Department of Nutrition Science, East Carolina University, 388 Ward Sports Medicine Building, Greenville, NC 27858, USA, Tel: 252-328-9414 (O), 717-870-9082 (C), E-mail: wentzl@ecu.edu
Int J Sports Exerc Med, IJSEM-2-034, (Volume 2, Issue 1), Original Article; ISSN: 2469-5718
Received: November 23, 2015 | Accepted: January 04, 2016 | Published: January 07, 2016
Citation: Hiserote AM, Berry-Cabán CS, Wu Q, Wentz LM (2016) Correlations between Vitamin D Concentrations and Lipid Panels in Active Duty and Veteran Military Personnel. Int J Sports Exerc Med 2:034. 10.23937/2469-5718/1510034
Copyright: © 2016 Hiserote AM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Vitamin D deficiency is common in the United States and has been associated with dyslipidemia along with additional cardiovascular conditions. Dyslipidemia raises the risk for cardiovascular disease and has been linked to symptoms of post-traumatic stress disorder (PTSD) in military personnel. The purpose of this study was to identify significant correlations between serum vitamin D and lipid panel concentrations in active duty and veteran military personnel. This analysis examined 3,053 unique cases of serum vitamin D and lipid panel assessments ordered at Womack Army Medical Center, Fort Bragg, North Carolina, from January 2012 to September 2013. Assessments were drawn within 21 days of one another. Fifty-seven percent of subjects had insufficient vitamin D status, and 36.6% had high total cholesterol according to the Army Medical Department guidelines, using 30 ng/ml for 25-hydroxyvitamin D and 200 mg cholesterol, respectifvely. In regression models, vitamin D was significantly positively correlated with high-density lipoprotein cholesterol in all subjects after controlling for age, gender and military status. Body mass index (BMI) was available for active personnel only, and including BMI values in analysis reduced the significance of vitamin D in the model. Vitamin D concentrations were negatively associated with total cholesterol and low-density lipoprotein in veterans only. Overall, our data suggest that lipoprotein concentrations are complex and may be influenced by vitamin D status in military service members. Therefore, future research should aim to explain the correlation between vitamin D and cholesterol concentrations in service members to determine if improving vitamin D status would also improve cholesterol status.
Keywords
Cholesterol, High-density lipoprotein, Low-density lipoprotein, 25-hydroxyvitamin D, Dyslipidemia, Service members
Abbreviations
AMDD: Army Medical Department, BMI: Body Mass Index, HDL-C: High-Density Lipoprotein Cholesterol, LDL-C: Low Density Lipoprotein Cholesterol, PTSD: Post-Traumatic Stress Disorder, TC: Total Cholesterol, TG: Triglycerides, US: United States, UV: Ultraviolet, 25(OH)D: 25-hydroxycholecalciferol
Introduction
Vitamin D functions as a hormone in the human body. The primary source of vitamin D is endogenous synthesis in response to solar ultraviolet (UV) radiation, although individuals also rely on dietary sources, especially in winter months and those in northern latitudes [1,2]. Dietary sources include fatty fish (salmon and herring), liver, eggs, and fortified foods, such as milk and other dairy products [3,4].
Worldwide, most humans expose less than 5% of their body to UV light for adequate time to synthesize vitamin D, increasing their risk for deficiencies if dietary requirements are not met [1]. People living at latitudes greater than 40 degrees are not exposed to adequate UV exposure during the winter months to replenish vitamin D concentrations [2]. Additionally, individuals with darker skin tones (non-White), who are older, who live in areas with urban photochemical smog or always wear sunscreen are at greater risk for inadequate vitamin D. As a result, vitamin D status. deficiency is widespread across civilian and military populations [5,6]. It has been estimated that between 25% and 57% of the United States (US) population are deficient in vitamin D [1,7,8].
Vitamin D has essential roles in calcium homeostasis, as well as regulation of cellular growth, function and differentiation [4]. Recent studies have identified vitamin D receptors in the liver, immune system, and skeletal and cardiac muscles, suggesting that vitamin D has widespread functions throughout the body [1]. Poor vitamin D status has been associated with diabetes, obesity, hypertension, peripheral vascular disease, coronary artery disease, stroke, insulin resistance, heart failure, and dyslipidemia [1,4,9-13].
Dyslipidemia is a known risk factor for cardiovascular disease, which is a major cause of morbidity and mortality worldwide [8,14]. Dyslipidemia has also been associated with psychological conditions, such as depression [15,16] and post-traumatic stress disorder (PTSD) in military personnel [17-24]. Since vitamin D deficiency has been linked to increased risk for dyslipidemia and depression, then vitamin D deficiency may also increase the risk for PTSD through its relationship with lipids [15,16,25].
Numerous studies have identified significant associations between 25-hydroxycholecalciferol (25(OH)D) concentrations and lipid profiles [13,26-30]. There are several potential mechanisms that connect vitamin D with lipid concentrations. Vitamin D may have a direct effect on serum lipid levels, such as affecting adipogenesis and differentiation, or vitamin D may have an indirect effect on serum lipid levels, such as through its effect on parathyroid hormone and calcium homeostasis [31]. It is also hypothesized that vitamin D may be transported by lipoproteins as vitamin D binding proteins have been discovered on lipoproteins, notably very low density lipoprotein (VLDL) [32]. Additionally, changes in levels of vitamin D binding protein have been shown to affect serum 25(OH)D levels [33]. Thus, it is plausible that circulating levels of lipoprotein have an effect on the levels of free 25(OH)D.
The purpose of this study was to identify significant correlations between serum vitamin D and lipid panel concentrations in active duty and veteran military personnel. Establishing a link between vitamin D status and dyslipidemia in service members is important to the treatment of cardiovascular disease and may play a role in reducing the risk for PTSD.
Materials and Methods
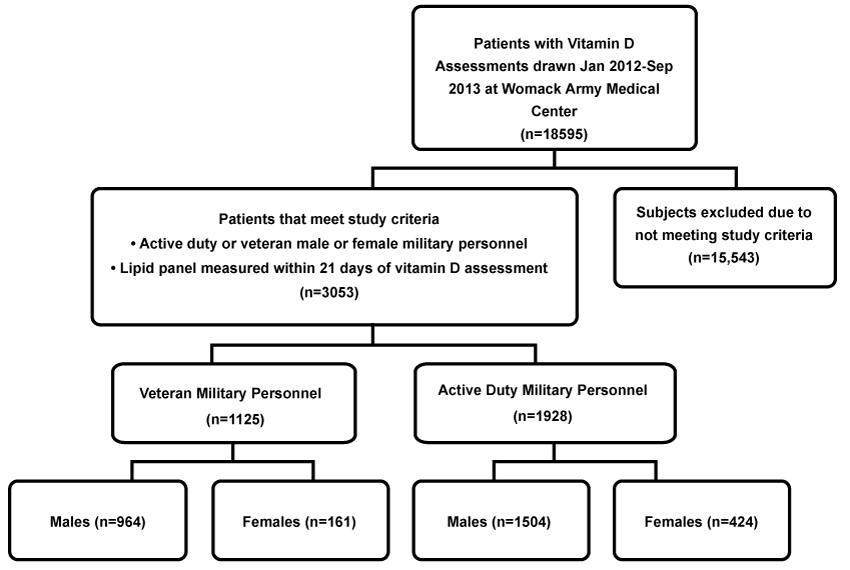
This retrospective study examined 3,053 unique cases of serum vitamin D assessments ordered at Womack Army Medical Center, Fort Bragg, North Carolina, between January 2012 and September 2013 (Figure 1). Inclusion criteria were active duty or veteran military personnel with a lipid panel measured within 21 days of vitamin D assessment. Age at the time of test as well as military status (active duty or veteran) was identified for all subjects, while body mass index (BMI) was available for only 1,350 active-duty subjects. Race and ethnicity identifiers were too limited to include in analysis.
Measurement of vitamin D and lipid panel assessments were conducted through Womack Army Medical Center. Serum 25(OH)D concentrations were determined by liquid chromatography-tandem mass spectrometry (Quest Diagnostics, Chantilly, VA) with a detection limit of 4 ng/ml and 8.3% coefficient of variation. Lipid concentrations were determined by enzymatic, colorimetric method (Roche Diagnostics, Indianapolis, IN) with a detection limit of 3.86 mg/dL and 1.6% coefficient of variation. Since serum 25(OH)D has a half-life of three weeks, subjects were included only if lipid panel assessment was conducted within 21 days of vitamin D assessment. Therefore, no adjustment for season was warranted.
Vitamin D and lipid concentrations were categorized according to the laboratory ranges used by the US Army Medical Department (AMEDD) standards of care [5]. The AMEDD laboratory ranges follow guidelines from the Endocrine Society Clinical Practice Guideline that defines deficient as 25(OH)D less than 20 ng/ml, insufficient as 20-29 ng/ml, and sufficient as 30-100 ng/ml [32]. AMEDD guidelines define high total cholesterol (TC) as > 200 mg/dL, above optimal low-density lipoprotein (LDL-C) as >130 mg/dL, high triglycerides (TG) as > 150 mg/dL, and low high-density lipoprotein (HDL-C) as < 35 mg/dL [5]. The BMI categories, underweight (< 18.5 kg/m2), normal (18.5-24.99 kg/m2), overweight (25.00-29.99 kg/m2), and obese (≥ 30.00 kg/m2) were defined based on the international classification of adult underweight, overweight and obesity according to BMI guidelines, published by the World Health Organization [34,35]. We recognize the controversary in defining vitamin D deficiency and have used AMEDD guidelines for all laboratory assessments since those were the ranges defined by the Army for this population.
Data were analyzed for descriptive statistics and correlations using SPSS version 20.0 (SPSS, Inc., Chicago, IL). Summary statistics for categorical variables included frequencies, means, and standard deviations. Marginal relationships between age, vitamin D, and HDL-C, LDL-C, TC and TG were assessed using Pearson's correlations. Student's t-test and ANOVA were used to compare means. General linear models were used to test the effect of vitamin D on lipid concentrations. In these models, age of soldiers was a covariate, and active duty vs. veteran was a fixed factor. BMI was included as a covariate in step-wise regression. Interactions were tested and removed from the models if they were not statistically significant at a level of p = 0.05. This study was approved by Womack Army Medical Center Institutional Review Board.
Results
We examined medical records for 3,053 subjects who met study criteria: 2,468 (80.8%) male and 585 (19.2%) females. Mean age was 45.8 ± 15.1 years; 1,125 (36.9%) of the subjects were veterans and 1,928 (63.1%) were active duty. The overall mean for 25(OH)D concentrations was 28.78 ± 11.72 ng/ml. Six-hundred twenty-three (20.4%) of subjects were vitamin D deficient; 1127 (36.9%) were vitamin D insufficient; and 1302 (42.7%) were vitamin D sufficient. High TC was found in 1118 (36.6%) of subjects; above optimal LDL-C was found in 778 (25.5%) of subjects; high triglyceride levels were found in 768 (25.8%) of subjects; and low HDL-C was found in 339 (11.1%) of subjects.
Table 1 shows service member age, lipid concentrations, and BMI according to 25(OH)D status. Age was significantly higher in the sufficient group than the deficient group. BMI was significantly higher in the deficient group than the sufficient group. TC and LDL-C were both significantly higher in the deficient group than the sufficient group. HDL-C was significantly higher in the sufficient group than the deficient group. TG were not significantly different between groups.
![]()
Table 1: Service Member age, lipid concentrations, and BMI according to 25-hydroxyvitamin D status.
View Table 1
Table 2 shows service member age, vitamin D concentrations, lipid concentrations, and BMI according to military status. Veterans were significantly older and had significantly higher 25(OH)D and TG concentrations than the active duty service members. Active duty service members had significantly higher TC, LDL-C, and HDL-C levels compared to veterans. BMI data were not available for veterans.
![]()
Table 2: Service Member age, vitamin D concentrations, lipid concentrations, and BMI according to military status.
View Table 2
Table 3 shows service member age, vitamin D concentrations, lipid concentrations, and BMI according to gender. Males were significantly older, had significantly higher BMI, LDL-C, and TG concentrations than females. Females had significantly higher HDL-C levels than males. There were no significant differences for 25(OH)D or TC concentrations.
![]()
Table 3: Service Member age, vitamin D concentrations, lipid concentrations, and BMI according to gender.
View Table 3
Table 4 shows service member age, vitamin D concentrations, and lipid concentrations according to BMI category for active duty service members. Subjects with obese BMI levels had significantly lower 25(OH)D concentrations than overweight and normal weight groups, while the overweight group had significantly lower levels than the normal weight group. HDL-C levels were significantly lower in the obese group than both the overweight and normal weight groups, while the overweight group also had significantly lower HDL-C levels than the normal weight group.
![]()
Table 4: Service Member age, vitamin D concentrations, and lipid concentrations according to BMI category.
View Table 4
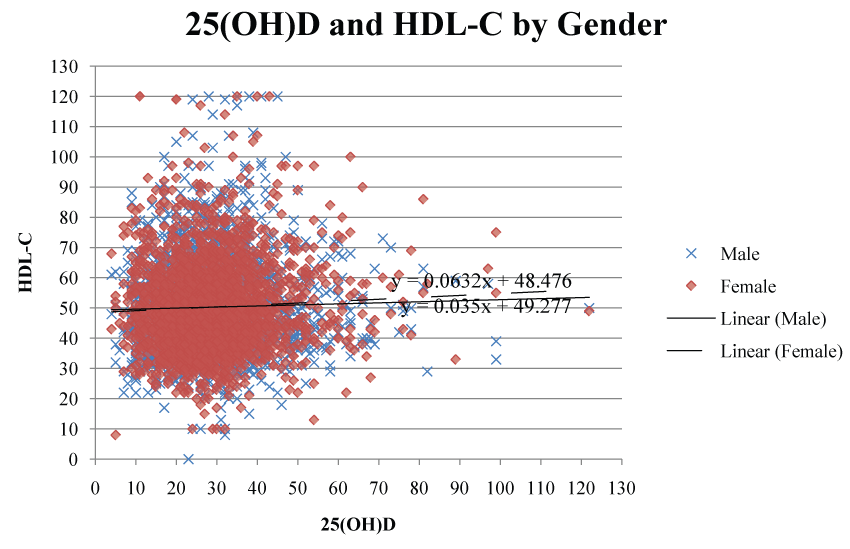
HDL-C levels were significantly positively correlated with 25(OH)D concentrations (r = 0.05112; p = 0.005). Serum 25(OH)D continued to be a significant predictor for HDL-C after adjustment for gender and military status (p = 0.0012) (Table 5). When examined by gender (Figure 2), serum 25(OH)D had a stronger correlation with HDL-C (r = 0.087; p = 0.038) in the female population than in the male population (r = 0.050; p = 0.013). However, adding BMI to the regression model in active duty subjects reduced the significance of 25(OH)D on HDL-C (p = 0.06). LDL-C and TC concentrations were significantly negatively correlated with 25(OH)D concentrations (p < 0.001; r = -0.08195 and p < 0.0001; r = -0.06004, respectively). After adjustment for military status, 25(OH)D had a significant negative linear effect on LDL-C and TC concentrations in veterans only (p < 0.001 and p = 0.0002 respectively). TG levels were not significantly correlated with 25(OH)D concentrations.
.
Figure 2: Scatter plot illustrating the correlation between 25(OH)D and HDL-C separated by gender.
View Figure 2
![]()
Table 5: General linear models to predict HDL-C levels in veteran and active duty military personnel.
View Table 5
Discussion
In this study, we found that the majority of service members tested for vitamin D had deficient/insufficient status. Furthermore, service members with low vitamin D had higher TC, higher LDL-C, and lower HDL-C compared to those with sufficient vitamin D. When adjusted for age, gender, and status, 25(OH)D had a significant negative linear effect on TC and LDL-C in the veteran population only, while 25(OH)D had a significant positive linear effect on HDL-C in active duty and veteran service members. The strongest relationship was identified between 25(OHD)D and HDL-C concentrations.
Overall, 20.4% of military personnel in our sample were vitamin D deficient, and 57.3% were deficient/insufficient, despite the southern latitude of 35.1°N. Data on the prevalence of vitamin D deficiency in the US military are limited, although vitamin D deficiency is common among the US population [36]. One study reported that 31.1-33.5% of serum samples from active duty service members had 25(OH)D less than 20ng/ml, when adjusted for season of sample analysis [37]. Another study found that 57% of female military recruits entering basic training had serum 25(OH)D concentrations less than 30ng/ml, and their 25(OH)D concentrations decreased after eight weeks of outdoor training in the Southeastern US [38]. Our results were consistent with both of these American military studies.
In our study, 36.6% subjects had high TC; 26.5% subjects had above optimal LDL-C; 22% subjects had low HDL-C; and 25.8% subjects had high TG. Current prevalence of dyslipidemia in military personnel in the US is lacking. In the general US adult population, it is estimated that 38.4% have high TC, 32.2% have high LDL-C, 25.1% have high TG, and 17% have low HDL-C based on statistics gathered from a variety of national surveys [39]. Prevalence of abnormal lipid panels in our sample is consistent with this analysis, although we found a larger prevalence of low HDL-C in our study sample. One military study analyzed autopsy results from US service members (n = 3,832) who died in support of Operations Enduring Freedom or Operation Iraqi Freedom/New Dawn (excluding cause of death labeled "suicide," "natural," or "undetermined"), to include analysis of atherosclerosis and dyslipidemia [40]. Coronary or aortic atherosclerosis was present in 12.1% of the service members, while less than 1% (n = 28) service members had dyslipidemia. Of those with dyslipidemia, 50% had atherosclerosis. Lastly, 166 (4.3%) of the service members were obese, according to their BMI. These findings were not consistent with our results, as we found higher prevalence of obesity in active duty members and higher rates of dyslipidemia in both active duty and veteran service members.
When adjusted for military status, vitamin D had a significant effect on both TC and LDL-C in veterans only. In our study population, the veteran group was significantly older, had significantly lower TC and LDL-C, and had significantly higher 25(OH)D concentrations than the active duty group. TC levels typically decrease with age by mechanisms not entirely understood [41]. It is hypothesized that this decrease results from the prevalence of chronic illness in the older population rather than the physiological changes associated with age. Like TC levels, vitamin D also has been shown to decrease with age [34]. Somewhat unexpectedly, our results showed that age was significantly greater in subjects with sufficient vitamin D status. Since veterans are free from active duty responsibilities and uniform requirements, they may have had more sunlight exposures, but since we did not have data on outdoor activity, this hypothesis cannot be confirmed.
In our sample, the strongest relationship was a positive correlation between vitamin D and HDL-C concentrations. Our findings of a significant positive association between serum 25(OH)D and HDL-C concentrations is consistent with other military and civilian publications [27,29,42]. In US civilian men and women, as 25(OH)D was a significant predictor for HDL-C after adjusting for age, sex, waist circumference, physical activity score, alcohol consumption, smoking, and vitamin D supplementation [27]. Furthermore, no significant associations were found between 25(OH)D and LDL-C or total cholesterol. In civilian Norwegian subjects 25 years and older, a significant positive association between 25(OH)D and HDL-C was identified and maintained over 13 years in this longitudinal analysis [47]. In Belgian military personnel, there was a significant positive correlation between serum 25(OH)D and HDL-C in women, but not in men [29]. Our data showed a postitive correlation between 25(OH)D and HDL-C in both men and women. One cause of this discrepancy could be the smaller sample size in the Belgian study (n = 358) compared to our much larger sample size (n = 3,053).
Furthermore, one intervention study found that supplementing vitamin D in deficient Indian children significantly improved HDL-C concentrations [43]. These authors hypothesize that vitamin D regulates cholesterol-carrying macrophages involved in reverse cholesterol transport, thus supporting a role in HDL-C synthesis. Alternativley, some researchers suggest that vitamin D may be a marker of chronic nonspecific illness rather than a direct contributor to any specific disease, noting that unhealthy people have vitamin D deficiency due to decreased exposure to sunlight [13]. However, unhealthy subjects have not supported the relationship between 25(OH)D and HDL-C, as type 2 diabetic civilian subjects from Iran did not have a significant positive association between 25(OH)D and HDL-C [44]
In our study, adding BMI to the active duty model reduced the significance of vitamin D on HDL-C in activity duty service members. Therefore, BMI likely explained much of the connection between 25(OH)D and HDL-C, since BMI was significantly negatively correlated with both 25(OH)D (r = 0.117; p < 0.001) and HDL-C (r = 0.683; p < 0.001) concentrations. Since vitamin D is fat soluble and is easily taken up into adipose tissue, individuals with more body fat have a greater storage capacity for vitamin D that could result in lower concentrations of circulating 25(OH)D [27,45]. Low vitamin D in obese individuals may also stem from these individuals having less motility or ability to participate in outdoor activities and thus have inadequate sun exposure [30]. Additionally, higher body fat has been associated with lower HDL-C concentrations [46]. However, other research has found that 25(OH)D continued to be a significant predictor for HDL-C when physical characteristics were included in the analysis [27].
The relationship between poor vitamin D status and dyslipidemia has implications beyond cardiovascular disease, as dyslipidemia has been associated with psychological conditions, including depression [26,27] and PTSD in military personnel [28-31,34-37]. Studies have shown that soldiers with combat-related PTSD have significantly higher TC, LDL-C, and TG and lower HDL-C levels compared to soldiers without PTSD [17,19,23,24]. Dyslipidemia was found in younger active duty military personnel (mean age 31) as well as older veterans [17,18,23]. It is hypothesized that dyslipidemia may increase activity of the noradrenergic system and thereby aggravate symptoms of PTSD [17], which is supported by a strong positive correlation between noradrenalin and lipid levels [23]. Additonally, civilian research has shown that vitamin D deficiency increases risk for cognitive deficits and depression, although no link has been established between vitamin D status and symptoms of PTSD [47]. Since evidence suggests that vitamin D deficiency increases the risk for dyslipidemia and depression, then vitamin D deficiency may also increase the risk for PTSD although this hypothesis requires further research [15,16,25].
Other factors such as diet and exercise may impact this observed relationship. Exercise has been shown to improve both serum vitamin D and lipid profile concentrations [48]. Plant-based diets, especially, vegan diets, have also been shown to improve serum lipid profile concentrations [49,50]. However, vitamin D is found in only small concentrations in plant-based foods, and vegans have been shown to have low serum vitamin D levels despite improved serum lipid profile concentrations [49,50]. While we did not have data on our subjects diet and exercise habit, a recent study on the US military found that 31.2% of soldiers fail to meet the recommended amount of moderate aerobic exercise per week (at least 150 minutes per week); 43% fail to meet the recommended amount of vigorous aerobic training (at least 75 minutes per week); and 53% fail to meet the recommended amount of strength training per week (at least three days per week) [51]. Furthermore, only 10.8% of soldiers eat at least three servings of fruit per week, and 12.9% eat at least three servings of vegetables per day [51]. These habits likely play a role in the serum vitamin D levels and lipid panel concentrations of the US military population.
This study has several limitations. First it is a retrospective analysis, and we had no access to data on sun exposure, vitamin D consumption, medical history or medication data (including hyperlipidemic treatments and vitamin D supplements) for subjects. Therefore, we were able to identify correlations but not causal relationships. BMI data were available for active duty subjects only, and BMI may not accurately represent true body composition in active military personnel. Finally, since the study only included military personnel with medically ordered vitamin D and lipid assessments, the sample may not be representative of the entire military population. The study was strengthened by including only assays completed within 3 weeks of one another to control for season of vitamin D analysis, as well as only including one location to control for latitude variances.
Future research should prospectively examine the prevalence of vitamin D deficiency and dyslipidemia in active duty and veteran military personnel. Furthermore, longitudinal analyses should establish clinically significant links between vitamin D status and lipid profiles and if these factor influence PTSD symptoms. Further research should also clarify mechanisms that connect altered lipid profiles and body fat with vitamin D deficiency. Lastly, intervention studies to treat subjects with vitamin D deficiency and dyslipidemia would better explain these hormonal and lipid metabolism relationships.
In conclusion, serum 25(OH)D was significantly positively associated with HDL-C in all subjects after controlling for age, gender and military status. However, including BMI values in active duty personnel reduced the significance of 25(OH)D. Serum 25(OH)D concentrations were negatively associated with LDL-C and TC in veterans only. Overall, our data suggest that lipoprotein concentrations are complex and may be influenced by vitamin D status in military service members. Therefore, future research should aim to explain the correlation between vitamin D and cholesterol concentrations in service members to determine if improving vitamin D status would also improve cholesterol status.
Acknowledgments
We appreciate the assistance of John Rehder from Womack Army Medical Center Information Management Department and Jerad Eldred from University of New Mexico School of Medicine for their roles in data collection. No funding was used to support this work.
References
-
Reddy Vanga S, Good M, Howard PA, Vacek JL (2010) Role of vitamin D in cardiovascular health. Am J Cardiol 106: 798-805.
-
Nadir MA, Szwejkowski BR, Witham MD (2010) Vitamin D and cardiovascular prevention. Cardiovasc Ther 28: e5-e12.
-
DeLuca HF (2004) Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr 80: S1689- S 1696.
-
Makariou S, Liberopoulos EN, Elisaf M, Challa A (2011) Novel roles of vitamin D in disease: what is new in 2011? Eur J Intern Med 22: 355-362.
-
United States Army Composite Health Care System (2013) Department of Defense Database. Womack Army Medical Center.
-
Wentz LM, Eldred JD, Henry MD, Berry-Caban CS (2014) Clinical relevance of optimizing vitamin d status in soldiers to enhance physical and cognitive performance. J Spec Oper Med 14: 58-66.
-
Abuannadi M, O'keefe JH (2011) Vitamin D and cardiovascular health. Prim Care Cardiovasc J 4: 59-62.
-
Parker J, Hashmi O, Dutton D, Mavrodaris A, Stranges S, et al. (2010) Levels of vitamin D and cardiometabolic disorders: systematic review and meta-analysis. Maturitas 65: 225-236.
-
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357: 266-281.
-
Zhou JC, Zhu YM, Guo P, Chen Z, Xie FZ, et al. (2013) Serum 25(OH)D and lipid levels in Chinese obese and normal weight males before and after oral vitamin D supplementation. Biomed Environ Sci 26: 801-807.
-
Chaudhuri JR, Mridula KR, Anamika A, Boddu DB, Misra PK, et al. (2013) Deficiency of 25-hydroxyvitamin d and dyslipidemia in Indian subjects. J Lipids 2013: 623420.
-
Cutillas-Marco E, Prosper AF, Grant WB, Morales-Suárez-Varela MM (2013) Vitamin D status and hypercholesterolemia in Spanish general population. Dermatoendocrinol 5: 358-362.
-
Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, et al. (2008) Vitamin D deficiency and risk of cardiovascular disease. Circulation 117: 503-511.
-
Kingsbury KJ, Bondy G (2003) Understanding the essentials of blood lipid metabolism. Prog Cardiovasc Nurs 18: 13-18.
-
Józefowicz O, Rabe-Jablonska J, Wozniacka A, Strzelecki D (2014) Analysis of vitamin D status in major depression. J Psychiatr Pract 20: 329-337.
-
Spedding S (2014) Vitamin D and depression: a systematic review and meta-analysis comparing studies with and without biological flaws. Nutrients 6: 1501-1518.
-
Kagan BL, Leskin G, Haas B, Wilkins J, Foy D (1999) Elevated lipid levels in Vietnam veterans with chronic posttraumatic stress disorder. Biol Psychiatry 45: 374-377.
-
Grenier JL, Swenson JR, FitzGibbon GM, Leach AJ (1997) Psychosocial aspects of coronary artery disease related to military patients. Can J Psychiatry 42: 176-184.
-
Karlovik D, Martinac M, Buljan D, Zoricik Z (2004) Relationship between serum lipid concentrations and posttraumatic stress disorder symptoms in soldiers with combat experiences. Acta Med Okayama 58: 23-27.
-
Jendricko T, Vidovik A, Grubisik-Ilik M, Romik Z, Kovacik Z, et al. (2009) Homocysteine and serum lipids concentration in male war veterans with posttraumatic stress disorder. Prog Neuropsychopharmacol Biol Psychiatry 33: 134-140.
-
Heppner PS, Crawford EF, Haji UA, Afari N, Hauger RL, et al. (2009) The association of posttraumatic stress disorder and metabolic syndrome: a study of increased health risk in veterans. BMC Med 7: 1.
-
Dzubur Kulenovik A, Kucukalik A, Malec D (2008) Changes in plasma lipid concentrations and risk of coronary artery disease in army veterans suffering from chronic posttraumatic stress disorder. Croat Med J 49: 506-514.
-
Karlovik D, Buljan D, Martinac M, Marcinko D (2004) Serum lipid concentrations in Croatian veterans with post-traumatic stress disorder,post-traumatic stress disorder comorbid with major depressive disorder,or major depressive disorder. J Korean Med Sci 19: 431-436.
-
Maia DB, Marmar CR, Mendlowicz MV, Metzler T, Nóbrega A, et al. (2008) Abnormal serum lipid profile in Brazilian police officers with post-traumatic stress disorder. J Affect Disord 107: 259-263.
-
Howland RH (2011) Vitamin D and depression. J Psychosoc Nurs Ment Health Serv 49: 15-18.
-
Karhapää P, Pihlajamäki J, Pörsti I, Kastarinen M, Mustonen J, et al. (2010) Diverse associations of 25-hydroxyvitamin D and 1,25-dihydroxy-vitamin D with dyslipidaemias. J Intern Med 268: 604-610.
-
Maki KC, Rubin MR, Wong LG, McManus JF, Jensen CD, et al. (2009) Serum 25-hydroxyvitamin D is independently associated with high-density lipoprotein cholesterol and the metabolic syndrome in men and women. J Clin Lipidol 3: 289-296.
-
Chung JY, Hong SH (2013) Vitamin D status and its association with cardiometabolic risk factors in Korean adults based on a 2008-2010 Korean National Health and Nutrition Examination Survey. Nutr Res Pract 7: 495-502.
-
Auwerx J, Bouillon R, Kesteloot H (1992) Relation between 25-hydroxyvitamin D3, apolipoprotein A-I, and high density lipoprotein cholesterol. Arterioscler Thromb 12: 671-674.
-
Botella-Carretero JI, Alvarez-Blasco F, Villafruela JJ, Balsa JA, Vázquez C, et al. (2007) Vitamin D deficiency is associated with the metabolic syndrome in morbid obesity. Clin Nutr 26: 573-580.
-
Jorde R, Grimnes G (2011) Vitamin D and metabolic health with special reference to the effect of vitamin D on serum lipids. Prog Lipid Res 50: 303-312.
-
Speeckaert MM, Taes YE, De Buyzere ML, Christophe AB, Kaufman JM, et al. (2010) Investigation of the potential association of vitamin D binding protein with lipoproteins. Ann Clin Biochem 47: 143-150.
-
Altinova AE, Ozkan C, Akturk M, Gulbahar O, Yalcin M1, et al. (2015) Vitamin D-binding protein and free vitamin D concentrations in acromegaly. Endocrine .
-
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, et al. (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96: 1911-1930.
-
(2000) Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 894: 1-253.
-
Ganji V, Zhang X, Tangpricha V (2012) Serum 25-hydroxyvitamin D concentrations and prevalence estimates of hypovitaminosis D in the U.S. population based on assay-adjusted data. J Nutr 142: 498-507.
-
Umhau JC, George DT, Heaney RP, Lewis MD, Ursano RJ, et al. (2013) Low vitamin D status and suicide: a case-control study of active duty military service members. PLoS One 8: e51543.
-
Andersen NE, Karl JP, Cable SJ, Williams KW, Rood JC, et al. (2010) Vitamin D status in female military personnel during combat training. J Int Soc Sports Nutr 7: 38.
-
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. (2015) Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation 131: e29-322.
-
Webber BJ, Seguin PG, Burnett DG, Clark LL, Otto JL (2012) Prevalence of and risk factors for autopsy-determined atherosclerosis among US service members, 2001-2011. JAMA 308: 2577-2583.
-
Volpato S, Zuliani G, Guralnik JM, Palmieri E, Fellin R (2001) The inverse association between age and cholesterol level among older patients: the role of poor health status. Gerontology 47: 36-45.
-
Jorde R, Figenschau Y, Hutchinson M, Emaus N, Grimnes G (2010) High serum 25-hydroxyvitamin D concentrations are associated with a favorable serum lipid profile. Eur J Clin Nutr 64: 1457-1464.
-
Hirschler V, Maccallini G, Sanchez MS, Castaño L, Molinari C (2013) Improvement in high-density lipoprotein cholesterol levels in argentine Indian school children after vitamin D supplementation. Horm Res Paediatr 80: 335-342.
-
Saedisomeolia A, Taheri E, Djalali M, Moghadam AM, Qorbani M (2014) Association between serum level of vitamin D and lipid profiles in type 2 diabetic patients in Iran. J Diabetes Metab Disord 13: 7.
-
Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF (2000) Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 72: 690-693.
-
Kazumi T, Kawaguchi A, Hozumi T, Ishida Y, Yoshino G (1997) Serum HDL cholesterol values are associated with apoB-containing lipoprotein metabolism and triglyceride-body fat interrelation in young Japanese men. Atherosclerosis 130: 93-100.
-
McCann JC, Ames BN (2008) Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J 22: 982-1001.
-
Chomistek AK, Chiuve SE, Jensen MK, Cook NR, Rimm EB (2011) Vigorous physical activity, mediating biomarkers, and risk of myocardial infarction. Med Sci Sports Exerc 43: 1884-1890.
-
Tuso PJ, Ismail MH, Ha BP, Bartolotto C (2013) Nutritional update for physicians: plant-based diets. Perm J 17: 61-66.
-
Craig WJ (2009) Health effects of vegan diets. Am J Clin Nutr 89: 1627S-1633S.
-
Army Public Health Center (2015) Health of the force report.