International Journal of Sports and Exercise Medicine
Characterization of Endorphin Gene Expression Following Electroporation in Mice Tibialis Anterior Muscle: Implications for Gene Doping Detection
Morten Klitgaard Nohr1 , Parisa Gazerani1,2*, Julie Gehl3 and Jacek Lichota1
1Section of Biomedicine, Department of Health Science and Technology, Aalborg University, Denmark
2Center for Sensory-Motor Interaction, Department of Health Science and Technology, Aalborg University, Denmark
3Center for Experimental Drug and Gene Electrotransfer, Department of Oncology, Copenhagen University Hospital Herlev, Denmark
*Corresponding author: Parisa Gazerani, Pharm D, PhD, Department of Health Science and Technology, Aalborg University, Frederik Bajers Vej 7D3, 9220 Aalborg East, Denmark, Tel: (+45) 9940 2412, Fax: (+45) 9815 4008, E-mail: gazerani@hst.aau.dk
Int J Sports Exerc Med, IJSEM-1-003, (Volume 1, Issue 1), Research Article; ISSN: 2469-5718
Received: March 16, 2015 | Accepted: April 10, 2015 | Published: April 13, 2015
Citation: Nohr MK, Gazerani P, Gehl J, Lichota J (2015) Characterization of Endorphin Gene Expression Following Electroporation in Mice Tibialis Anterior Muscle: Implications for Gene Doping Detection. Int J Sports Exerc Med 1:003. 10.23937/2469-5718/1510003
Copyright: © 2015 Nohr MK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Since ancient time, doping has been used by athletes. The prevalence for using performance-enhancing agents is increasing and new methods are being applied. Gene doping is foreseen to become one of the new doping methods in athletic performances. This study was therefore performed to investigate the possibility of transfecting a gene encoding the endogenous peptide preproenkephalin (PENK) in mice as gene doping candidate and subsequently establish a functional detection method. This was performed using the blood and muscle as samples mimicking the most plausible detection method to be used in real life. Our results showed that it is possible to transfect PENK into mice and subsequently detect it.. However, it was only possible to detect PENK in the blood after 24 hours and in the muscle after 48. This therefore illustrates the trouble in detecting the use of gene doping, as the blood sample must be acquired no longer than 24 hours after transfection. The gene doping detection method must therefore be implemented as a normal routine check, if the antidoping agencies have a chance against the athlete's willingness to use doping.
Introduction
Since ancient time, doping has been used by athletes for a better chance of winning. The prevalence has been increasing since, thus showing the willingness athletes possess for using performance-enhancing agents. In the professional sports a total of 66 % was excluded by the U.S. Anti-Doping Agency (USADA) because of the use of doping [1], and 3% of the non-professional fitness users have either used or use anabolic steroids [2].
Different types of performance-enhancing agents are used in different branches of sports, e.g. erythropoietin is widely used in cycling and testosterone in weightlifting competitions. The world's anti-doping agency (WADA) has therefore formed a list of doping agents, which contains all substances and methods that can potentially be used as performance-enhancing agents. On this list a relatively new doping method has been added, which is gene doping. This is a technique, in which a gene of interest is inserted into either the cell nucleus transiently or even permanently in the genome of the athlete [3]. The inserted gene is capable of producing the desired protein, thus the gene doping user may receive the same effect as using conventional doping, but with a reduced risk of efficient detection and thus disqualification.
In order to insert a gene, different methods have been applied. One of these is called electroporation [4] and exhibits potential as a method for gene doping.
In a recent study, the researchers showed that plasmid DNA encoding erythropoietin (EPO) could successfully be transfected into muscle in mice and rats, and skin in mice [5,6]. This was confirmed by measuring the levels of EPO in blood samples. The results showed an increase in EPO, which reached to the desirable therapeutic level [5,6]. This indicates that it is possible to insert a gene of interest and obtain a measurable effect, which could be a therapeutic method for helping patients with protein deficiencies.
In addition to EPO, a number of other genes could potentially be applied for doping purposes. One of the potential candidates would be insertion of a gene that encodes enkephalins. The effect would contribute to a higher pain tolerance while performing heavy sports related tasks. Enkephalins are neuropeptides, which bind to opioid receptors in the nervous system, causing analgesic effects [7]. This was confirmed by a study conducted by JR. Goss et al, which showed that an inserted gene encoding PENK into dorsal root ganglion of rats exerted an analgesic effect [8]. Therefore, the evidence is clear for a method of treating pain, but also way of suppressing pain under exercise. It is only a matter of time before athletes could undertake this new doping method by a proper pain reducing gene and application of an effective transfection method.
Therefore, the antidoping agencies should consider acquiring a technique of detecting of this doping method.
Electroporation based gene delivery is now in experimental use for the treatment of e.g. cancer [9]
The present study is focusing on the insertion of a gene encoding preproenkephalin (PENK) into muscle cells of mice, and subsequently attempting to detect this gene in the blood and muscles by quantitative polymerase chain reaction (qPCR). The transfection method is electroporation, as this is the most possible method for athletes to use due to its safety and efficiency [10].
Materials and Methods
Animals
All animal experiments were conducted in agreement with the recommendation of the European Convention for the protection of Vertebrate Animals used for Experimentation. The experiments were approved by the animal ethics committee (2013-15-2937-00760) and performed at Herlev Hospital, Denmark. A total of sixteen, 6-8 weeks old female C57BL/6 mice were used.
The animals were bred at Herlev Hospital, and maintained in a thermo-stated environment under a 12-hour light/dark cycle and had free access to food and drinking water.
Plasmid and transformation
The plasmid used in this experiment was purchased from Origene, USA (Cat. MG203555). The plasmid contained the sequence encoding fusion protein with mouse PENK (pubmed ref: NM_001002927.2) in frame with green-fluorescent protein (GFP) thereby enabling visualization of a successful transfection and subcellular localization of PENK.
The plasmid was transformed by heat-shock into the chemically competent E. coli strain DH5α (Invitrogen, USA), and plated onto an agar plate containing 100μg/ml ampicillin. Bacteria were then incubated for 24 hours, resulting in the formation of colonies. One colony was used to propagate and purify the necessary amount of plasmid by a plasmid purification kit, NucleoSpin Midi (Machery-Nagel, Germany) for further experiments.
In vitro experiment
Cell culture: C2C12 myoblast cells (ATCC, USA) were cultivated in medium consisting of DMEM (Sigma Aldrich, USA) with 10 % fetal calf serum (Gibco, USA) and 1 % penicillin/streptomycin (Gibco, Cat. No. 15140-122). Cells were grown in T175 culture flask. When cell confluence reached 60-70 %, cells were trypsinized and washed twice with sterile PBS. The cells were counted on a hemocytometer (Assistant, Germany) and passage into a new flask with fresh medium.
Chemical transfection: When 50% of cell confluence was reached the cells were trypsinized, counted and seeded into a 6-well culture plate at 2500 cells/cm2. Cells were then allowed to reach a confluence of 70–90% (24 hours), before transfection with the Turbofect® transfection reagent (Thermo Scientific, USA). This step was performed using 2μg plasmid DNA according to manufacurer's protocol. Control wells were handled likewise with the exception of Turbofect® transfection reagent (Thermo Scientific, USA).
The transfection rate was calculated as percentage of transfected cells/total number of cells on five representative pictures taken from the same transfection.
Confocal microscopy: After visual confirmation of transfection using inverted fluorescent microscope (Axiovert 40, Carl Zeiss Microscopy, Germany) , the cells were trypsinized, resuspended and seeded into a chamber slide (C6307, Sigma-Aldrich, USA). The cells were then left for 4 hours, allowing them to attach to the glass slide. They were then fixed with 4% paraformaldehyde, and stained with 4',6-diamidino-2-phenylindole (DAPI) . Hereafter, cells were visualised on a confocal microscopy (LSM 700, Carl Zeiss Microscopy, Germany).
In vivo experiment
Mice (n=16) were anaesthetised 15 min prior to electroporation by injection of 100µL of Hypnorm/Midazolam combination (2,5ml hypnorm/2,5ml Midazolam mixed with 5ml sterile water) subcutaneously. 20μl of plasmid solution (containing 10μg plasmid) was injected intramuscularly, using a 29G insulin syringe, into the musculus tibialis anterior. After the injection a plate electrode with a 4mm gap in between were fitted around the leg, which was shaved beforehand. Electrode gel was applied to ensure sufficient contact. A control group received 20μl of sterile saline injection instead of plasmid solution.
The electric field was applied using the Cliniporator (IGEA, Italy), which applied a combination of a High voltage pulse (800V/cm (applied voltage=320V), 100μs) and a low voltage pulse (100V/cm (applied voltage=40 V), 400μs) with a 1 sec pause between pulses [6].
RNA extraction and qPCR analysis: The mice were euthanized by quick cervical dislocation, followed by decapitation and blood was collected in EDTA-containing eppendorf tubes. The right musculus tibialis anterior were removed and quickly frozen in liquid nitrogen.
Total RNA was extracted from 1ml whole blood samples using QIAamp RNA blood mini kit (Qiagen, Germany). The RNA concentration was measured using Nanophotometer (Implen, Germany).
Total RNA was extracted from 30mg muscle samples using AllPrep DNA/RNA Mini kit (Qiagen, Germany) or RNeasy Fibrous Tissue mini kit (Qiagen, Germany) according to the manufacturer's protocol. All muscle tissue was homogenized in liquid nitrogen using pestle and mortar.
cDNA synthesis was performed using the RevertAid™ Premium First Strand cDNA synthesis kit (Thermo Scientific, USA). This was done using the provided oligo (dT)18 and random hexamer primers following protocol by the manufacturer.
Following primers for qPCR analysis were used:
PENK: Fwd GTCCTGCCTCCTGGCTACAGTG
Rev TCCAGTGTGCACGCCAGGAAAT;
β-actin: Fwd CCTCTGAACCCTAAGGCCAACCGTGAA
Rev AGTGGTACGACCAGAGGCATACAGG.
qPCR was performed using 1µl cDNA, 10µL Brilliant SYBR Green qPCR Master mix (Agilent Technologies, Denmark) and 0,4µM of each primer. qPCR was done using MX3000P® instrument (Agilent Technologies, Denmark). All samples were run in duplicates.
Water and RT- samples were used as negative controls. The thermal profile for qPCR was 1×: 95°C 10 min; 40×: 95°C 30 sec, 61°C 10 sec, 70°C 30 sec; 1×: 95°C 1 min, 55°C 30sec, 95°C 30sec
Statistics
All PENK expression data were normalised to β-actin, and the data are presented as fold to this. Statistical analysis and visual presentation were performed using GraphPad Prism (GraphPad Software, USA). Kruskal-Wallis test followed by multiple comparisons between the individual groups was performed. The error bars in figure 2 and 3 represent SEM. p values less than 0.05 was considered significant.
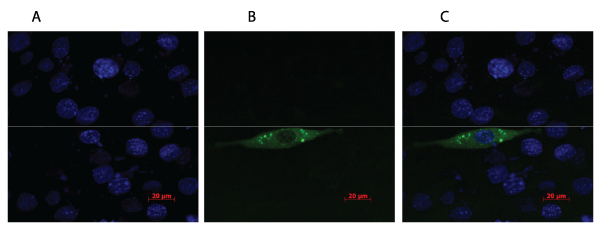
.
Figure 1: C2C12 myoblast cells stained with DAPI (A) and transfected with plasmid expressing the fusion protein GFP-PENK (B). Green fluorescent light
illustrates the GFP-PENK fusion protein and the blue colour presents the cells stained with DAPI. The fusion protein is localized in small vesicles near the
periphery of the cell (C), which is expected as PENK secreted into the blood.
View Figure 1
Results
To evaluate if the PENK plasmid could be efficiently expressed in muscle cells, C2C12 cells were transfected with a plasmid expressing PENK fused with green fluorescent protein (GFP). As indicated in Figure 1, GFP-PENK and DAPI positive cells were observed, and were found in the same focal plane. DAPI allows visualization of the nuclei of cells and was used to estimate the ratio between successfully transfected cells and non-transfected cells.
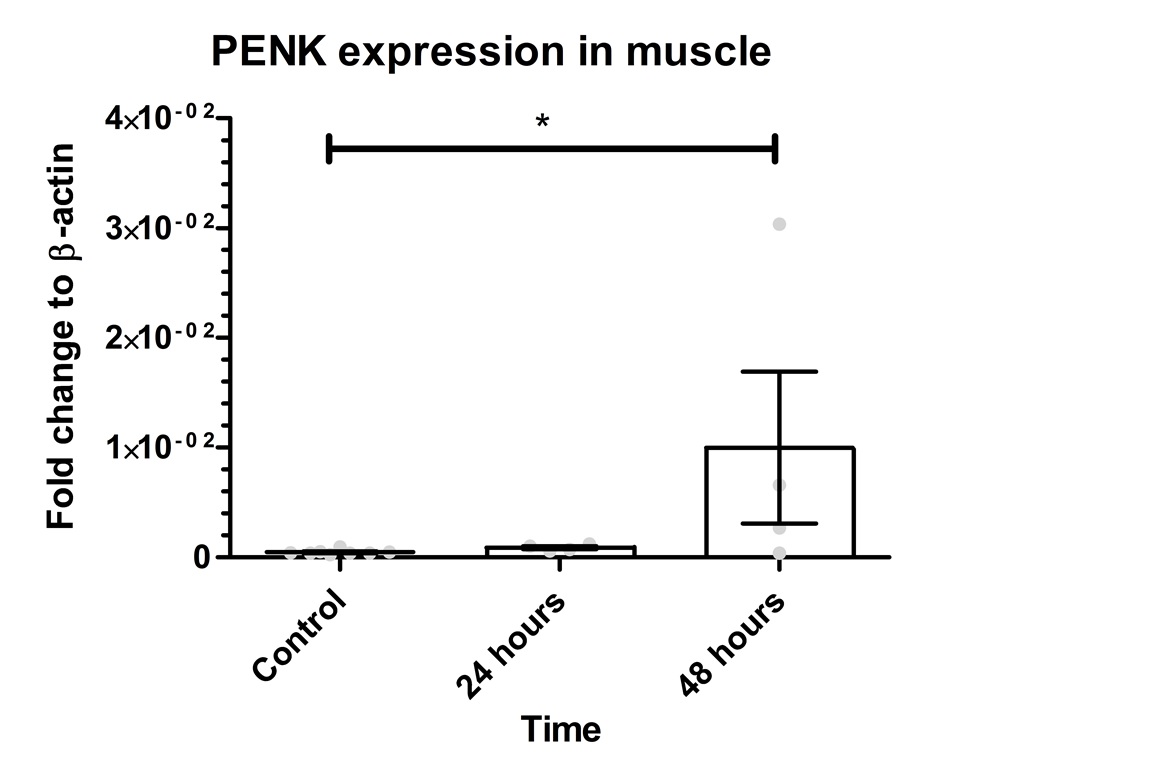
.
Figure 2: PENK gene expression in the muscle. The graph shows mean values for PENK gene expression as 0.0471*10-2 for control, 0.08805*10-2 for 24 hours, and 1*10-2 for 48 hours. The Kruskal-Wallis test revealed a significant difference between the groups (p=0.0297). There was a significant difference between the control group and 48 hours (p=0.0162). However, there was no significant difference between control group vs. 24 hours (p=0.0727) or 24 hours vs. 48 hours (p=0.3429). Error bars indicate SEM and grey dots show the individual data points. * represents p< 0.05.
View Figure 2
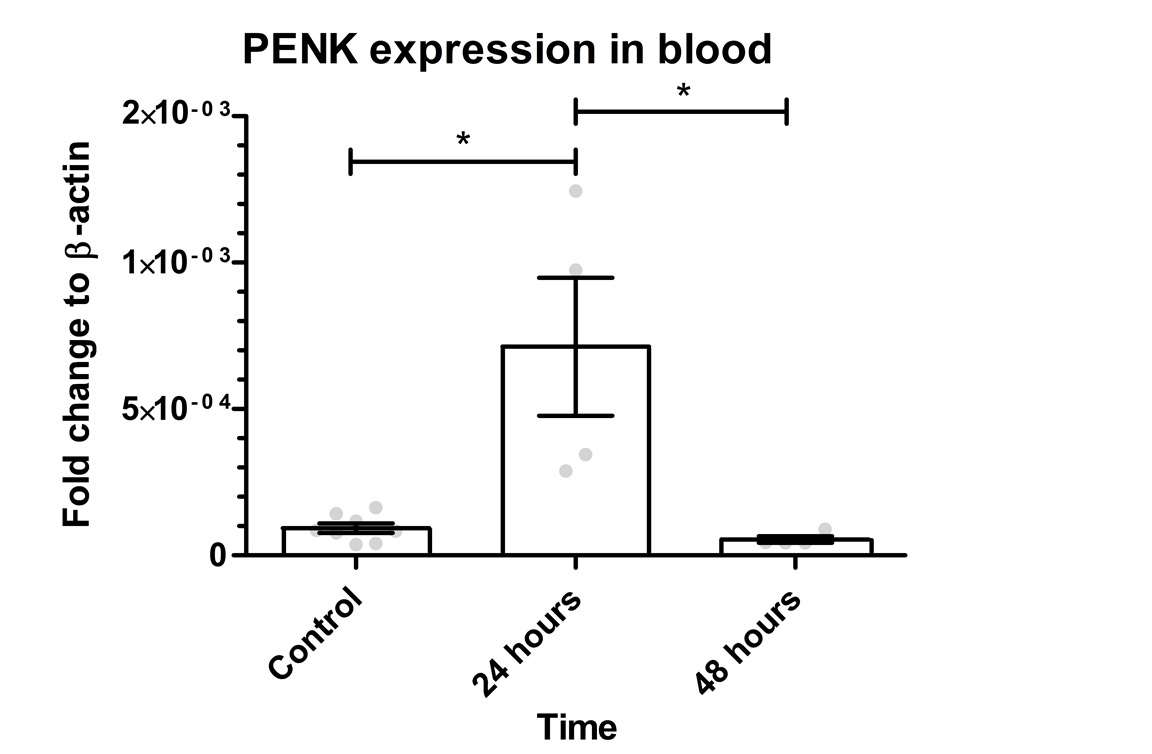
.
Figure 3: PENK gene expression in the blood. The graph shows mean values for PENK gene expression as 0.009297*10-2 for the control, 0.0713*10-2 for 24 hours, and 0.005456*10-2 for 48 hours. The Kruskal-Wallis test revealed a significant difference between the groups (p=0.0035). There was a significant difference between control group vs. 24 hours (p=0.0228) and 24 hours vs. 48 hours (p=0.0120). There was no significant difference between control vs. 48 hours (p=0.4606). Error bars indicate SEM and grey dots show the individual data points. * represents p< 0.05.
View Figure 3
Small vesicles with an intense GFP signal were localised in the peripheries of the cells, indicating that the GFP-PENK fusion protein resides in secretory granules as is the case with the native PENK-protein. The overall expression efficiency was determined to be 7%.
PENK transgene expression in muscle
To determine if PENK transgene could be detected in the blood and muscle sixteen C57BL/6 mice were transfected by electroporation with the same plasmid as in the in vitro experiment. Mice were decapitated after 24 and 48 hours and the blood as well as the right quadriceps muscle were collected. The PENK gene expression in the muscle was investigated by qPCR (Figure 2) showing a mean value of 0.0471*10-2 for control, 0.08805 *10-2 for 24 hours and 1*10-2 for 48 hours. The 24 hours group showed 1.868 times higher expression compared to control and 21.21 times higher after 48 hours. PENK 48 was 11.35 higher than PENK 24. The Kruskal-Wallis resulted with a p-value of 0.0297 between groups. There was a significant difference between the control group vs. 48 hours (p=0.0162).
PENK transgene expression detected in blood samples
The same gene expression analysis was done for the blood samples. This was to test whether the GFP-PENK transgene is detectable in the whole blood after muscle electroporation. As shown in Figure 3, the mean value for PENK gene expression is 0.009297*10-2 for the control, 0.0713*10-2 for 24 hours and 0.005456*10-2 for 48 hours. The 24 hours group showed 7.67 times higher expression compared to control and 0.58 times lower after 48 hours. PENK 24 was 13.07 higher than PENK 48. The Kruskal-Wallis test showed a p-value of 0.0035 between groups. There was a significant difference between the control group vs. 24 hours (p=0.0228) and 24 hours vs. 48 hours (p=0.0120).
Discussion
Doping remains a major challenge, and the number of athletes and novices being disqualified from sport competitions due to illegal agents [2], illustrates the importance for an effective and easy detection method. In this study, we show that it is possible to transfect a gene doping candidate (PENK) into the muscle of of mice, using electroporation. and subsequently a method for detecting this illicit gene.
Cultured C2C12 muscle cells were used in the first place to validate the plasmid and define subcellular localisation of GFP-PENK fusion protein. GFP expression was observed in C2C12 cells transfected with the GFP-PENK plasmid. GFP signal was visible in small clusters, close to the cell membrane, which suggests that the fusion protein is located in vesicles or secretory granules. This corresponds with the fact that PENK normally is secreted from cells [11]. Transfection efficiency was estimated to be 7 %, which was lower than reported by others (60 - 80 %) [12]. However, the goal of this experiment was to confirm that GFP-PENK could be expressed in muscle cells, and since a clear GFP expression was seen, the low transfection efficiency in this experiment was not an issue. These data indicate that it is possible to transfect plasmid in the muscle cells, and make them produce GFP-PENK fusion protein. The modern doping methods can easily move into direction of gene-transfer, as the technology is available and relatively simple. The reason for choosing electroporation in the project is the fact that chemical transfection method will be troublesome to use as a doping method in vivo in contrary to electrotransfer [13]. Some studies indicate that the success rate of chemical transfection in vivo is very low, which can be due to many factors including nucleic acid/chemical ratio, solution pH and cell membrane conditions [14]. On the other hand, this kind of transfection method offers a reliable transfection method for in vitro experiments.
In our in vivo muscle transfection model the GFP-PENK expression was 1.868 fold higher 24 hours post-transfection compared to control muscle and 21.21 fold higher 48h post-transfection. The statistical analysis showed a significant difference between the groups. These findings correlate with previous studies, showing it is possible to transfect and obtain sufficient transcription after gene insertion using electroporation as transfection method [6].
These findings suggest that the transcription of GFP-PENK in the transfected muscle maintains at least for 48 hours, which means that it is possible to detect a transgene of interest in the muscle of gene doping suspects, even with small amount of analysis material. A previous study indicates that inserted transgene in the muscle was still being transcribed and translated into proteins months after electroporation [6]. The problem for the anti-doping agencies would be to locate the transgenic muscle cells [15], with the lack of cooperation from the athlete.
After a successful transfection and expression of the transgene it was interesting to investigate the possibility of transgene detection in the blood. The hypothesis was that during electroporation some portion of the blood must be co-transfected with the muscle, and with a potential leak of blood from the transfected muscle, it is possible to detect the transgenic agents in a blood sample. If that is the case and the transgene is detectable in the blood it would make a detection method far easier. According to our findings it is possible to detect GFP-PENK fusion gene expression in the blood 24 hours after electroporation. However the expression drops dramatically already 48 hours after electroporation.
The transgene needs to be transfected into the leukocytes for an efficient expression. However, mature white blood cells have a half-life of 7 hours [16], and might therefore perish before the transgene expression can be detected. The possibility of detecting the transgenic cells is therefore troublesome after 48 hours, which indicates that the antidoping agencies must require a blood sample from the athletes within 24 hours after the use of gene doping if the gene doping should be detectable in the blood sample.
As mentioned earlier the insertion of the gene encoding erythropoietin (EPO) using electroporation as transfection method, has been capable of producing a therapeutic effect [6,15]. The same result has to be confirmed with PENK, before this gene can be fully classified as a gene doping candidate. In addition to this it would be necessary to establish a dose-response curve on the amount of plasmid encoding PENK and the PENK protein produced to obtain a significant analgesic effect in the athlete's muscle.
However, if higher amounts of plasmid are required to produce a desirable analgesic effect, this could cause toxicity problems for the survival of the transfected cells [17]. According to a study by Chuang et al. [18] 40μg of PENK plasmid was enough to produce an analgesic effect in rats, therefore is seems that high amounts are needed to produce pain relief [19].
Previous studies indicate that it is of pivotal importance to use an efficient transfection method when inserting a gene into the genome [3]. This study is therefore offering a real method of inserting a gene of interest, but also an effective method of transgene detection in the blood sample. The evidence is therefore strong that gene doping is a real risk, but the results of this study propose a method of the doping detection.
Electroporation is the most plausible gene doping method, compared the other transfection methods. According to other studies, electroporation provides a safe and efficient method for the insertion of a gene of interest into muscle cells [5,6,15]. According to the findings of this study [15], only cells directly within the electrical field would be transfected. The chemical transfection reagents on the contrary offer high transfection efficiency, but their action is not localized to a specific tissue, which could lead to unwanted side effects [20]. Viral vectors are capable of integrating the gene of interest into the genome [21]. However, with viral transduction the transcription can become stable and therefore securing a more permanent gene doping effect [8]. On the contrary, viral transduction will cause an immune response and the production of antibodies [22], which can serve as another detection method. Concluding, many different methods have been applied as effective gene delivery tools but the question remains, which method will be used in the real life. The most successful method will most probably ensure the longest duration of expression of the transgenic material. As Hojman et al. [5] showed the transgenic EPO gene was producing a therapeutic effect after 42 days post-transfection, which means that a gene doped athlete would need a “booster” of the transgenic materiel for maintenance of the beneficial effect.
Conclusion
This study indicates that it is possible to transfect plasmid encoding GFP-PENK into the muscle and achieves a stable expression for 48 hours. It was also possible to detect the transgene in the muscle tissue up to 48 hours after transfection whereas in the blood sample the duration of transgene expression was limited to 24 hours posing a challenge for anti-doping agencies. Henceforth, it is impossible to locate the transfection site, and detect the illegal gene in the blood, this gene doping method may revolutionise the sport community negatively.
Acknowledgements
We would like to thank Jesper Franch and Pascal Madeleine for their contribution in application for funding for this study. We also thank the staff and members of the oncology section and associated laboratories, especially Stine Krog Frandsen and Anne Boye at Herlev hospital for their excellent assistance. Technical assistance from Jeppe Lund Nielsen from Department of Biotechnology, Chemistry and Environmental Engineering for confocal microscopy is highly appreciated. This project has been carried out with the support of Anti-Doping Denmark.
References
-
AGENCY, USADA. [http://www.usada.org/testing-statistics]
-
Anti Doping Danmark (2013) Forside.
-
Wang Z, Troilo PJ, Wang X, Griffiths TG, Pacchione SJ, et al. (2004) Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Ther 11: 711-721.
-
Bodles-Brakhop AM, Heller R, Draghia-Akli R (2009) Electroporation for the delivery of DNA-based vaccines and immunotherapeutics: current clinical developments. Mol Ther 17: 585-592.
-
Hojman P, Gissel H, Gehl J (2007) Sensitive and precise regulation of haemoglobin after gene transfer of erythropoietin to muscle tissue using electroporation. Gene Ther 14: 950-959.
-
Gothelf A, Hojman P, Gehl J (2010) Therapeutic levels of erythropoietin (EPO) achieved after gene electrotransfer to skin in mice. Gene Ther 17: 1077-1084.
-
Anna Baoutina, Ian E Alexander, John EJ Rasko, Kerry R Emslie (2007) Potential use of gene transfer in athletic performance enhancement. Mol Ther 10: 1751–1766
-
Goss JR, Mata M, Goins WF, Wu HH, Glorioso JC, et al. (2001) Antinociceptive effect of a genomic herpes simplex virus-based vector expressing human proenkephalin in rat dorsal root ganglion. Gene Ther 8: 551-556.
-
Spanggaard I, Snoj M, Cavalcanti A, Bouquet C, Sersa G, et al. (2013) Gene electrotransfer of plasmid antiangiogenic metargidin peptide (AMEP) in disseminated melanoma: safety and efficacy results of a phase I first-in-man study. Hum Gene Ther Clin Dev 24: 99-107.
-
Terova G, Rimoldi S, Bernardini G, Saroglia M (2013) Inhibition of myostatin gene expression in skeletal muscle of fish by in vivo electrically mediated dsRNA and shRNAi delivery. Mol Biotechnol 54: 673-684.
-
Hall J. E (2011) Textbook of medical physiology (12th edn).
-
TurboFect Transfection Reagent (2013) Thermo Scientific.
-
Kee ST, Gehl J, Lee EW (2011) Clinical Aspects of Electroporation.
-
Tae Kyung Kim, James H. Eberwine (2010) Mammalian cell transfection: the present and the future. Anal Bioanal Chem 397: 3173-3178.
-
Spanggaard I, Corydon T, Hojman P, Gissel H, Dagnaes-Hansen F, et al. (2012) Spatial distribution of transgenic protein after gene electrotransfer to porcine muscle. Hum Gene Ther Methods 23: 387-392.
-
Walker HK, Hall WD, Hurst JW (1990) Clinical Methods The History, Physical, and Laboratory Examinations. 3rd Edition Butterworth Publishers USA.
-
Lundsted D (2012) Trypsin-induced VEGF expression in adipose-derived stem cells; optimization of assays.
-
Chuang IC, Jhao CM, Yang CH, Chang HC, Wang CW, et al. (2004) Intramuscular electroporation with the pro-opiomelanocortin gene in rat adjuvant arthritis. Arthritis Res Ther 6: R7-7R14.
-
Chuang YC, Yang LC, Chiang PH, Kang HY, Ma WL, et al. (2005) Gene gun particle encoding preproenkephalin cDNA produces analgesia against capsaicin-induced bladder pain in rats. Urology 65: 804-810.
-
Felgner JH, Kumar R, Sridhar CN, Wheeler CJ, Tsai YJ, et al. (1994) Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. J Biol Chem 269: 2550-2561.
-
Gonçalves MA (2005) Adeno-associated virus: from defective virus to effective vector. Virol J 2: 43.
-
Halbert CL, Standaert TA, Aitken ML, Alexander IE, Russell DW, et al. (1997) Transduction by adeno-associated virus vectors in the rabbit airway: efficiency, persistence, and readministration. J Virol 71: 5932-5941.





