International Journal of Sports and Exercise Medicine
Electromyographic Cross-Spectral Analysis of Antagonist Muscle Coactivation
Travis W Beck*
Department of Health and Exercise Science, University of Oklahoma, USA
*Corresponding author: Travis W Beck, Associate Professor, Director, Biophysics Laboratory, Department of Health and Exercise Science, University of Oklahoma, 110 Huston Huffman Center, Norman, OK, USA, Tel: 405- 325-1378, E-mail: tbeck@ou.edu
Int J Sports Exerc Med, IJSEM-1-001, (Volume 1, Issue 1), Research Article; ISSN: 2469-5718
Received: February 25, 2015 | Accepted: March 16, 2015 | Published: March 20, 2015
Citation: Beck TW (2015) Electromyographic Cross-Spectral Analysis of Antagonist Muscle Coactivation. Int J Sports Exerc Med 1:001. 10.23937/2469-5718/1510001
Copyright: © 2015 Beck TW. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The purpose of this study was two-fold: (1) to compare the responses for traditional EMG amplitude measures with those from the cross-spectrum approach for assessing agonist-antagonist interaction, and (2) to examine changes in the agonist-antagonist interaction for the elbow flexors and extensors with increases in movement velocity. Eighteen healthy, college-aged subjects (mean ± SD age = 21.8 ± 2.7 yrs) volunteered to perform maximal, concentric isokinetic contractions of the dominant elbow flexors and extensors at randomly ordered velocities of 60, 180, and 300 degrees/second. The subjects also performed maximal, 6-sec isometric contractions of the dominant elbow flexors and extensors at an elbow joint angle of 120 degrees between the arm and forearm. During all contractions, bipolar surface EMG signals were detected from the biceps brachii and triceps brachii. Traditional EMG amplitude (root-mean-square, RMS) measurements were made for both the agonist and antagonist muscles during each contraction. In addition, total power and mean frequency (MNF) were calculated from the agonist-antagonist muscle cross-spectrum. The results indicated that there were no velocity-related changes in traditional EMG amplitude for either the agonist or antagonist muscles. There were also velocity related changes in cross-spectral power, but not MNF, for both elbow flexion and elbow extension. Mean cross-spectral power values were similar when comparing elbow flexion versus elbow extension during isometric contractions. During the dynamic contractions, however, mean cross-spectral power values were lower (relative to the isometric values) at all velocities for elbow extension, but higher at all velocities for elbow flexion. These findings suggested that the agonist-antagonist cross-spectrum approach may provide information regarding agonist-antagonist interaction that cannot be obtained from traditional EMG amplitude measurements. In addition, the agonist-antagonist interaction may be different for elbow flexion versus elbow extension.
Introduction
Antagonist muscle coactivation refers to light/moderate contraction of an antagonist muscle during agonist muscle activity. This phenomenon occurs during both strong contractions [1], as well as with lighter activity (e.g., walking) [2]. At first, antagonist muscle coactivation seems counter-productive, since it reduces net torque production at the joint. However, this phenomenon has a variety of functions, the most important of which include maintaining joint stability, properly distributing forces to prevent excessive wear of articular cartilage, and precise control of limbs in situations where a great deal of movement accuracy is needed [1,3,4]. The amount of antagonist muscle coactivation, as well as its timing, is generally assessed with surface electromyography (EMG). Of special importance when using surface EMG to assess coactivation is cross-talk (i.e., contamination of the EMG signal from the muscle of interest with another signal from a separate muscle) [5]. If the two muscles of interest are sufficiently separated, cross-talk is generally not a concern. However, if the muscles are close to each other, cross-talk can be problematic, and special procedures must be used to reduce its influence (e.g., careful consideration of electrode placement and special signal processing procedures).
Examining antagonist muscle coactivation during dynamic contractions can also be challenging, considering that the amount and timing of coactivation can change across the range of motion. For example, [6] reported increased levels of antagonist coactivation in both the quadriceps femoris and hamstring muscle groups, but only for the last 40 degrees of the range of motion during isokinetic knee flexion and extension contractions, respectively. It was suggested that these increased levels of coactivation were important for decelerating the leg near the end of the range of motion [6]. These findings [6] suggest, then, that antagonist coactivation should be greater at high velocities than at low velocities due to the need for greater limb deceleration at higher speeds. Interestingly, the evidence on this issue is conflicting. Bazzucchi et al. [1] reported no changes in antagonist coactivation with increases in velocity for either the biceps brachii or triceps brachii during maximal isokinetic elbow flexion and extension contractions at velocities ranging from 15-240 degrees/second. Hagood et al. [6], however, reported velocity-related increases in coactivation for both the quadriceps femoris and hamstring muscle groups, but not at all joint angles. These conflicting findings [1,6] underline the importance of analyzing consistent parts of the range of motion when examining antagonist muscle coactivation.
There is also evidence to suggest that there may be muscle-specific differences in antagonist coactivation. Osternig et al. [7] found much higher levels of antagonist coactivation from the hamstrings during high speed isokinetic knee extension than from the quadriceps femoris muscles during isokinetic knee flexion. To that end, it has been suggested [8] that the primary purpose of antagonist coactivation is not only to maintain joint stability, but also to equalize the load distribution in the joint to prevent excessive wear and tear of articular cartilage. If this is the case, then it would only be logical to conclude that different muscle groups would have different levels of antagonist coactivation, given the anatomical differences between joints. Although Bazzucchi et al. [1] examined potential velocity-related changes in antagonist muscle coactivation for the elbow flexors and extensors, traditional EMG amplitude was the only variable measured. There is certainly nothing wrong with this methodology, as it has provided much information on both the timing and amount of antagonist coactivation for many years. However, advancements in signal processing techniques have allowed researchers to extract more information from the EMG signal than ever before. One of these developments is in cross-spectral analysis [9], which allows researchers to identify the commonalities present in two separate signals. Specifically, cross-correlation of the two signals of interest, followed by a Fourier Transform of the cross-correlation function, yields a cross-spectrum that provides information regarding the amount of common information present in the signal and the frequency distribution(s) of this information [9]. In theory, an increase in agonist-antagonist cross-spectral power should reflect more commonalities between the two signals; which is potentially a very different situation (with a different underlying physiological mechanism) from a simple increase in antagonist coactivation. No previous investigations, however, have used cross-spectral analysis to examine agonist-antagonist interaction, and if this interaction changes with increases in movement velocity. Therefore, the purpose of this study was two-fold: (1) to compare the responses for traditional EMG amplitude measures with those from the cross-spectrum approach for assessing agonist-antagonist interaction, and (2) to examine changes in the agonist-antagonist interaction for the elbow flexors and extensors with increases in movement velocity. We hypothesize that the cross-spectral analysis will provide new information regarding the agonist-antagonist interaction that cannot be obtained from traditional EMG amplitude measures. In addition, there will be both muscle-specific and velocity-related differences in the agonist-antagonist interaction.
Methods
Subjects
Seventeen men (mean ± SD age=21.9 ± 2.8 yrs, height=181.1 ± 6.2 cm, body weight=78.8 ± 10.1 kg) and one woman (age=20.0 yrs, height=180.34 cm, body weight=81.7 kg) volunteered to participate in this investigation. The study was approved by the University Institutional Review Board for Human Subjects, and all subjects signed an informed consent form and completed a health history questionnaire prior to testing. All subjects were recreationally active, and reported no current or recent neuromuscular or musculoskeletal disorders that could have affected the outcome of the study.
Familiarization
The subjects were familiarized with the strength testing procedures during the first visit to the laboratory. Specifically, the subjects were tested for isometric and concentric isokinetic strength of their dominant (based on throwing preference) elbow flexors and extensors on a calibrated Cybex II isokinetic dynamometer. For all contractions, the subjects were positioned in front of the dynamometer on the upper body exercise testing table (UBXT) in accordance with the Cybex II User’s Manual (Figure 1). The isometric contractions were performed at a constant elbow joint angle of 120 degrees between the arm and the forearm, and were held for duration of six seconds. The concentric isokinetic contractions were performed at randomly ordered velocities of 60, 180, and 300 degrees/second. Prior to the isometric and concentric isokinetic contractions at each velocity, the subjects performed five submaximal contractions as a warm-up and to get the subjects accustomed to the movement velocity. Following these submaximal contractions, the subjects performed three separate maximal contractions for the strength assessments. The maximal isometric contractions were separated by two minutes of rest. The three separate concentric isokinetic contractions were performed in succession (i.e., flexion/extension, flexion/extension, flexion/extension), and two minutes of rest were allowed between testing at each velocity. The purpose of the familiarization session was only to provide the subjects with practice for the subsequent strength testing protocol to be performed on a separate day. Thus, no data were collected during the familiarization session.

Figure 1: Example of the positioning of the subject on the UBXT testing table for elbow flexion/extension.
View Figure 1
Strength testing
The subjects returned to the laboratory following at least 48 hours of rest to perform the strength testing. For this testing, the subjects performed the isometric and concentric isokinetic contractions of the dominant elbow flexors and extensors in exactly the same manner as during the familiarization session. Specifically, the five warm-up contractions were followed by three maximal contractions at each of the randomly ordered velocities. Two minutes of rest were allowed between the contractions at each velocity to ensure that the subjects were not fatigued during the testing.
EMG measurements
Surface EMG signals were detected from the biceps brachii and triceps brachii muscles during the maximal isometric and concentric isokinetic contractions with two separate bipolar (20mm center-to-center) surface electrode (circular 4 mm diameter silver/silver chloride, Biopac Systems, Inc., Santa Barbara, CA) arrangements. For each muscle, the bipolar electrode arrangements were placed in accordance with the recommendations of the SENIAM project (1999), and the reference electrodes were placed over the anterior distal end of the forearm between the styloid processes of the radius and ulna. The skin at each electrode site was prepared by careful shaving and cleansing with rubbing alcohol. The EMG signals from each muscle were amplified (gain: × 1,000) using differential amplifiers (EMG 100, Biopac Systems, Inc., Santa Barbara, CA).
Signal processing
The raw EMG signals were digitized at a sampling rate of 1,000samples/second and stored in a personal computer (Macintosh 7100/80 AV Power PC, Apple Computer, Inc., Cupertino, CA) for subsequent analysis. All signal processing was performed using custom programs written with Lab VIEW programming software (version 8.0, National Instruments, Austin, TX). All EMG signals were first bandpass filtered (fourth-order, zero lag Butterworth) at 10-500 Hz, and selected for the middle third of the contraction that generated the highest torque value. Specifically, for each isometric contraction, the EMG amplitude values were calculated for a 2.0 second time period corresponding to the middle 33% of the 6-sec isometric contraction. For each isokinetic contraction, the EMG amplitude values were calculated for a time period that corresponded to a 30 degree range of motion from approximately 120 to 150 degrees of elbow flexion. Thus, at 60 degrees/second, the amplitude values were calculated for a 0.5-sec segment. At 180 and 300 degrees/second, the amplitude values were calculated for 0.167-sec and 0.1-sec segments, respectively. This portion of the range of motion was selected at each velocity to avoid the acceleration and deceleration phases of movement which are typical of isokinetic dynamometers.
For each contraction, the root-mean-square (RMS) values of the selected EMG signals were calculated as a measure of EMG amplitude. The selected EMG signals from the biceps brachii and triceps brachii were then cross-correlated, and the Fourier Transform of the resulting cross-correlation function was calculated. The result of this Fourier Transformation is the cross-spectrum (Figure 2), which is similar in shape to the power spectrum. However, the cross-spectrum shows the amount of power shared by the two signals that were cross-correlated, and the frequencies where this power is located. Cross-spectral power was then determined by calculating the integral of the cross-spectrum. The mean frequency (MNF) of the cross-spectrum was also calculated as a measure of center frequency.
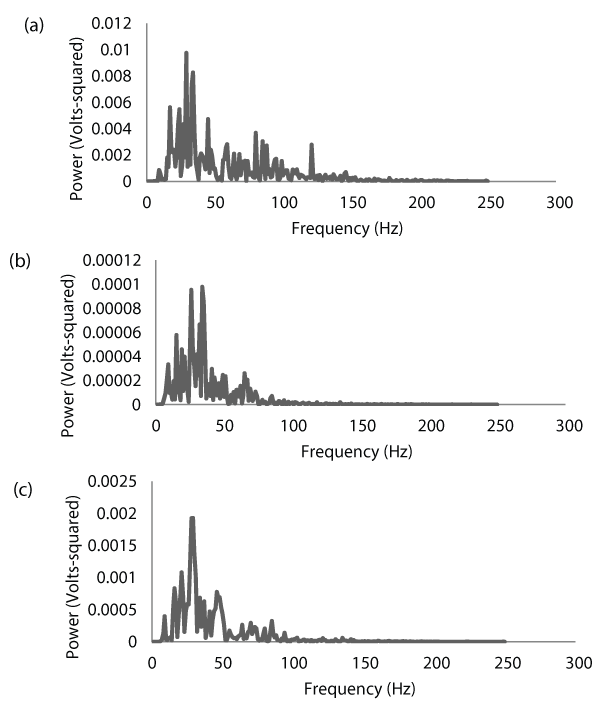
Figure 2: Electromyographic (EMG) power spectra for the biceps brachii (a)
and triceps brachii (b) of one subject during a maximal concentric isokinetic
muscle action of the forearm flexors at a velocity of 60 degrees/second. The
corresponding cross-spectrum between the two muscles is shown in (c).
Notice that the EMG signal from the biceps brachii (agonist) is approximately
100 times stronger than that of the triceps brachii (antagonist). Also notice
how the cross-spectrum highlights the coherence between the two signals.
View Figure 2
Statistical analyses
Four separate two-way (velocity × contraction type) repeated measures analyses of variance (ANOVAs) were used to analyze the EMG amplitude, cross-spectral power, and cross-spectral MNF data. When appropriate, follow-up analyses included one-way repeated measures ANOVAs and paired-samples t-tests. When a two-way interaction was present, Cohen’s d effect sizes were calculated for each pairwise comparison, with 95% confidence interval adjustments for the changes in the mean values. An alpha level of 0.05 was used to determine statistical significance for all comparisons.
Results
EMG amplitude
The results from the two-way repeated measures ANOVAs for EMG amplitude indicated that there were no significant velocity × contraction type interactions for either the biceps brachii or the triceps brachii (Figure 3). There were also no significant main effects for velocity, but there were significant main effects for contraction type. The marginal mean (collapsed across velocities) EMG amplitude value for the biceps brachii was greater during elbow flexion than the corresponding value during elbow extension. Similarly, the marginal mean (collapsed across velocities) EMG amplitude value for the triceps brachii was greater during elbow extension than during elbow flexion.
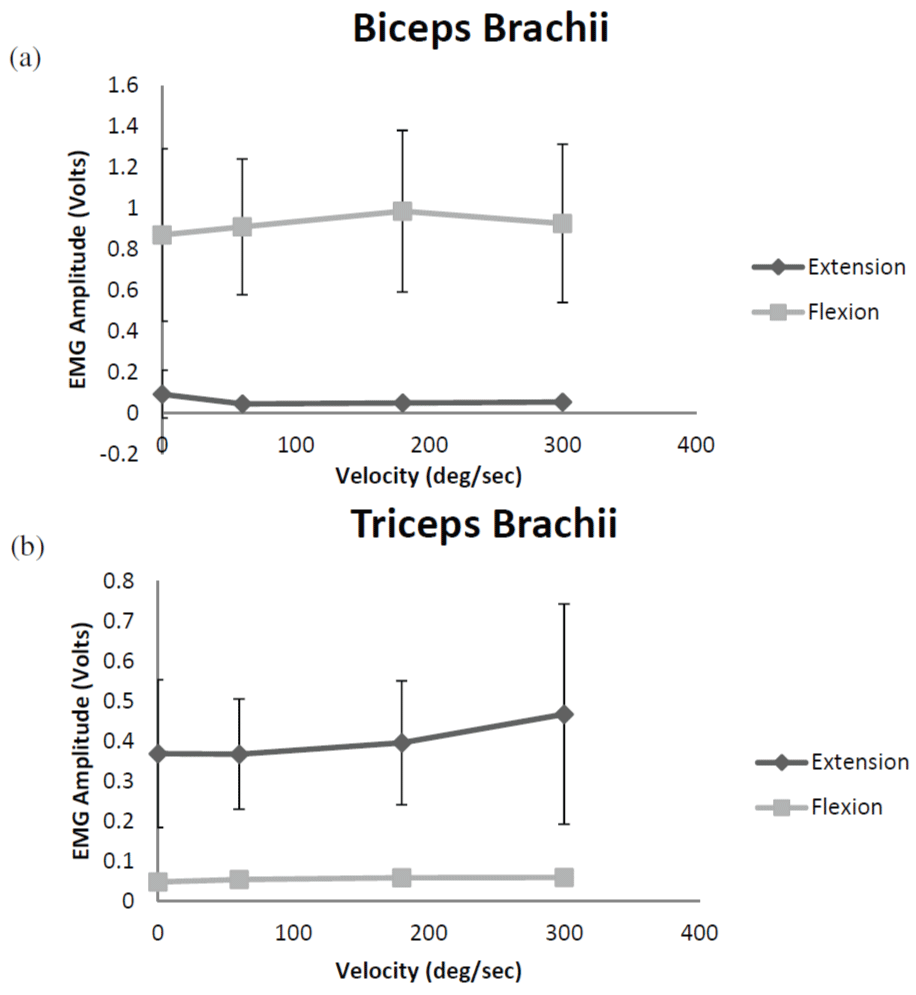
Figure 3: Electromyographic (EMG) amplitude for the biceps brachii (a) and
triceps brachii (b) during forearm flexion and extension. Values shown are
mean ± SD. Note the much lower values for the antagonist muscle, as well
as the relatively small changes in EMG amplitude with increases in velocity.
View Figure 3
Cross-spectral power
The results from the two-way repeated measures ANOVA for cross-spectral power indicated that there was a significant velocity × contraction type interaction (Figure 4a). Follow-up one-way repeated measures ANOVAs indicated that there were no significant changes in the mean cross-spectral power values with increases in velocity during elbow extension. However, the mean cross-spectral power values increased with velocity during elbow flexion (Figure 4a). The results from the paired-samples t-tests indicated that the mean cross-spectral power values during elbow flexion were significantly greater than those during elbow extension at 60, 180, and 300 degrees/second, but there was no mean difference during the isometric contraction (0 degrees/second). Table 1 shows the effect sizes (Cohen’s D) and 95% confidence interval adjustments for the differences between the mean cross-spectral power values for elbow flexion versus elbow extension. Notice the small effect size for the isometric contraction (0 degrees/second) and the large effect sizes during the dynamic contractions at all velocities.

Figure 4: (a) Electromyographic (EMG) cross-spectral power during forearm
flexion and extension. Values shown are mean ± SD. Note the relatively similar
values for forearm flexion and forearm extension during the isometric muscle
actions (0 deg/sec). During the dynamic muscle actions, however, crossspectral
power was greater for forearm flexion than for forearm extension at
all velocities. (b) Marginal mean (collapsed across contraction types) crossspectral
mean frequency (MNF) values with increases in velocity.
View Figure 4
![]()
Table 1: Cohen’s D effect sizes for the differences in cross-spectral power between flexion and extension at each velocity. The mean difference in cross spectral power (± 95% confidence interval) at each velocity is also shown.
View Table 1
Cross-spectral MNF
The results from the two-way repeated measures ANOVA for cross-spectral MNF indicated that there was no significant velocity × contraction type interaction (Figure 4b). There was also no significant main effect for contraction type, but there was a significant main effect for velocity. Follow-up analyses indicated that the marginal mean (collapsed across contraction types) cross-spectral MNF values increased with increases in velocity up to 180 degrees/second (Figure 4b).
Discussion
The data from the present study provided several very important findings. First, the traditional method of measuring antagonist EMG amplitude suggested that there were no changes in coactivation with increases in velocity from 0-300 degrees/second for either the biceps brachii or the triceps brachii. This would suggest that increases in movement velocity had no effect on antagonist muscle co activation. However, the cross-spectral analysis data did not support this conclusion. Although the mean cross-spectral power values were similar for elbow flexion and elbow extension during the isometric contractions, there was a large velocity-related dissociation between the responses during the dynamic contractions. Specifically, the mean cross-spectral power values for elbow flexion were greater than those for elbow extension at all velocities, with no mean differences between velocities (Figure 4a). In addition, the mean cross-spectral MNF values increased with velocity up to 180 degrees/second, but there were no mean differences between elbow flexion and elbow extension (Figure 4b).
To our knowledge, this is the first study to use cross-spectral analysis to examine antagonist muscle coactivation. Our findings demonstrated differences between elbow flexion and elbow extension for the amount of shared power in the EMG signal, but only during the isokinetic contractions. Thus, these results supported increased synergy (relative to isometric contractions) between the biceps brachii and triceps brachii during dynamic elbow flexion, but decreased synergy during dynamic elbow extension. Bazzucchi et al. [1] used traditional EMG amplitude measures to examine antagonist muscle coactivation in the elbow flexors and extensors during maximal isometric and concentric isokinetic contractions. Like the present study, the authors [1] found that antagonist EMG amplitude did not increase with velocity, although it was generally greater for the triceps brachii than for the biceps brachii. It was suggested [1] that triceps brachii activity may be more important for maintaining elbow joint stability during elbow flexion when compared to the biceps brachii during elbow extension. Hagood et al. [6] also examined the effect of movement velocity on antagonist muscle coactivation, although the quadriceps femoris and hamstring muscle groups were examined. The authors [6] reported significant increases in antagonist EMG amplitude for both the quadriceps femoris and hamstring muscles with increases in velocity from 15-240 degrees/second. However, this increase was specific to the final 40 degrees of the range of motion. During the first part of the range of motion, there were actually decreases in antagonist EMG amplitude with increases in velocity [6]. It was suggested Hagood et al. [6] that the velocity-related increase in antagonist coactivation was due to unintentional muscle activity for the purpose of improving knee joint stiffness.
The divergent patterns for cross-spectral power with increases in velocity for elbow flexion versus elbow extension are also interesting (Figure 4a). These findings suggested that when compared to isometric contractions, there is an increase in agonist-antagonist shared power during dynamic elbow flexion, but a decrease during dynamic elbow extension. These findings are interesting from a movement analysis perspective, and are likely due to: (1) the effects of gravitational acceleration on the limb, (2) differences in elbow flexion vs. elbow extension strength, and/or (3) the anatomical structure of the elbow joint. For example, the subjects in the present study performed the elbow flexion and extension contractions while lying on the UBXT in the supine position (in accordance with the procedures described in the Cybex II User’s Manual). When in this posture, (as well as the seated or standing postures), elbow extension is assisted by gravitational acceleration, while elbow flexion is counteracted. It is also possible that the greater cross-spectral power during elbow flexion than during elbow extension is simply due to more torque at the elbow joint. Although it was not analyzed statistically in this study, elbow extension peak torque was generally about 2/3 of the elbow flexion peak torque. In addition, every subject that participated in the study demonstrated greater peak torque values at all velocities for elbow flexion than for elbow extension. If an important role of antagonist coactivation is indeed to maintain joint stability [1], then it would be logical to postulate that greater coactivation should take place in situations where there is more torque. It is also possible, however, that the anatomical structure of the elbow joint affected the amount of antagonist coactivation required during elbow flexion vs. elbow extension. Specifically, [1] suggested that the “mechanical stop” provided by the olecranon process may provide added stability to the elbow joint during elbow extension, and particularly so at high velocities. Such a mechanical stop does not exist to halt the elbow flexion movement, thereby leading to an increased importance of triceps brachii activation to decelerate the elbow when it is near full flexion.
In conclusion, our results demonstrated two very important findings. First, the agonist-antagonist cross-spectral approach provides useful information about the interaction between agonist and antagonist muscles that cannot always be obtained from traditional measures of EMG amplitude. Second, elbow flexion demonstrated a greater agonist-antagonist interaction than elbow extension, but only during dynamic contractions. We hypothesize that these movement-related differences in antagonist muscle co activation could have been due to differential effects of gravitational acceleration on the elbow during elbow flexion versus elbow extension, greater torque at the elbow joint during elbow flexion than elbow extension, and/or the mechanical stop provided by the olecranon process to decelerate the elbow during elbow extension. Future studies should use the cross-spectral analysis to investigate these issues in more detail, as well as with different muscles and during various types of activities, including walking and running.
References
-
Bazzucchi I, Sbriccoli P, Marzattinocci G, Felici F (2006) Coactivation of the elbow antagonist muscles is not affected by the speed of movement in isokinetic exercise. Muscle Nerve 33: 191-199.
-
Hortobágyi T, Finch A, Solnik S, Rider P, DeVita P (2011) Association between muscle activation and metabolic cost of walking in young and old adults. The journals of gerontology. Series A, Biological sciences and medical sciences 66: 541-547.
-
Basmajian JV (1977) Motor learning and control: a working hypothesis. Arch Phys Med Rehabil 58: 38-41.
-
Enoka RM (1997) Neural strategies in the control of muscle force. Muscle Nerve Suppl 5: S66-69.
-
Morrenhof JW, Abbink HJ (1985) Cross-correlation and cross-talk in surface electromyography. Electromyogr Clin Neurophysiol 25: 73-79.
-
Hagood S, Solomonow M, Baratta R, Zhou BH, D'Ambrosia R (1990) The effect of joint velocity on the contribution of the antagonist musculature to knee stiffness and laxity. Am J Sports Med 18: 182-187.
-
Osternig LR, Hamill J, Lander JE, Robertson R (1986) Co-activation of sprinter and distance runner muscles in isokinetic exercise. Med Sci Sports Exerc 18: 431-435.
-
Solomonow M, Baratta R, Zhou BH, D'Ambrosia R (1988) Electromyogram coactivation patterns of the elbow antagonist muscles during slow isokinetic movement. Exp Neurol 100: 470-477.
-
Orizio C (1992) Soundmyogram and EMG cross-spectrum during exhausting isometric contractions in humans. J Electromyogr Kinesiol 2: 141-149.





