International Journal of Sports and Exercise Medicine
Effect of Hypercapnic Severity on Plasma Ammonia Accumulation and Respiratory Exchange Ratio during Incremental Exercise
Takahide Kato1*, Takaaki Matsumoto2, Atsuko Tsukanaka3, Masataka Nakano4, Ryo Ito5, Masato Amano6, Marc Cole7 and Stanley M Yamashiro7
1Department of General Education, National Institute of Technology, Toyota College, Toyota 471-8525, Japan
2Laboratory for Exercise Physiology and Biomechanics, School of Health and Sport Sciences, Chukyo University, Toyota 470-0393, Japan
3Department of Health and Nutrition, Kyoto Koka Women's University, Kyoto 615-0882, Japan
4Faculty of Human Study, Aichi Toho University, Nagoya 465-8515, Japan
5Department of Economics, Nihon Fukushi University, Chita 470-3295, Japan
6Trident College of Sports, Medical Care and Nursing, Nagoya 464-8611, Japan
7Department of Biomedical Engineering, Viterbi School of Engineering, University of Southern California, Los Angeles CA 90089, USA
*Corresponding author: Takahide Kato, Department of General Education, National Institute of Technology, Toyota College, 2-1 Eisei-cho, Toyota, Aichi 471-8525, Japan, Tel: +81-565-32-8811; Fax: +81-565-32-1872, E-mail: tkato@toyota-ct.ac.jp
Int J Sports Exerc Med, IJSEM-1-014, (Volume 1, Issue 3), Original Investigation; ISSN: 2469-5718
Received: June 29, 2015 | Accepted: July 17, 2015 | Published: July 20, 2015
Citation: Kato T, Matsumoto T, Tsukanaka A, Nakano M, Ito R, et al. (2015) Effect of Hypercapnic Severity on Plasma Ammonia Accumulation and Respiratory Exchange Ratio during Incremental Exercise. Int J Sports Exerc Med 1:014. 10.23937/2469-5718/1510014
Copyright: © 2015 Kato T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
We tested the effect of hypercapnic severity on plasma ammonia (Am) accumulation and respiratory exchange ratio (RER) during incremental exercise. Eight male subjects performed incremental cycle exercise to exhaustion under three conditions: breathing (1) Air, (2) 3% CO2 or (3) 6% CO2. Am in both hypercapnic conditions was lower than Air at 100% (P<0.05) without changing Am threshold (AmT). Am for 6% CO2 was lower than 3% CO2 immediately after exercise (P<0.05). RER in both hypercapnic conditions was lower than Air throughout the experiment (P<0.05). RER for 6% CO2 was lower than 3% CO2 at rest and 30% (P<0.05). RER for 3% CO2 at 30% was 0.74 without increasing arterialized blood partial pressure of CO2, which was near 0.7 (100% fat metabolism). AmT was unchanged by hypercapnic severity and suppression of AM accumulation was observed above AmT. Lowered RER during inhalation of 3% CO2 and 30% exercise occurred under isocapnic conditions, implying a change in metabolism. 3% CO2 and 30% was found to be the most effective combination for promoting fat metabolism. We demonstrate that Am accumulation and RER decrease with increasing hypercapnia.
Keywords
Hypercapnia, Ammonia, Respiratory exchange ratio, CO2 storage, Exercise
Introduction
Blood lactate (La) and ammonia (Am) concentrations are widely accepted markers of metabolism not only in clinical studies but also during exercise. Elevations from normal resting levels of both are observed in exercise and associated with deleterious effects such as fatigue. A decrease in blood La concentration during exercise under hypercapnic conditions was previously reported [1-4], and La threshold (LT) was unchanged by hypercapnia [4]. Further, we reported that plasma Am concentration was reduced at maximal exercise in a 6% inhaled CO2 condition [5]. However, the changes in Am dynamics during exercise, including Am threshold (AmT), remain unknown since blood samples were drawn following exercise. Interestingly, a previous study of exercise under hypercapnic conditions of 2, 4, and 6% CO2 demonstrated that blood La concentration during steady state exercise was the lowest at CO2 concentrations of 6% in inspired gas [2]. Thus, it is hypothesized that Am accumulation during exercise may be decreased depending on the severity of hypercapnia. Whether this is true has never been previously explored experimentally.
Also, hypercapnic gas inhalation causes a change in respiratory exchange ratio (RER). Previous studies reported a lowered RER during exercise under hypercapnia [2,6]. Substrate utilization during exercise can be estimated from the RER value and a low RER is expected to promote fat metabolism. Thus, a lowering of RER by hypercapnic gas inhalation may promote fat metabolism during exercise. Concerning the role of exercise training intensity on substrate utilization, fat metabolism is best promoted at exercise intensity less than 45% maximal oxygen uptake () [7]. However, the previous reports [2,6] used 45% or greater intensity, so CO2 inhalation and exercise intensity under 45% for reduction of RER is untried. Moreover, the changes in RER are usually complicated by changes in body carbon dioxide (CO2) stores tied to changes in partial pressure of arterial CO2 (). A decrease in RER could be due to a transient increase in the amount of CO2 stored in tissue due to an increased . Arterial CO2 concentration is approximately linearly proportional to and, when multiplied by cardiac output, leads to a predicted linearly proportional decrease in expired CO2 flux due to this increase in CO2 storage. Therefore, to apply a hypercapnic gas inhalation and show a change in fat metabolism, it is required to consider CO2 storage. An unchanging during RER measurement is the primary requirement.
The aims of the present study were to investigate: (1) the hypothesis that plasma Am concentration would decrease with increasing CO2 concentration in inhaled gas and (2) the effect of hypercapnic severity and relative exercise intensity on RER and CO2 storage. To examine these aims, we compared the effects of incremental exhaustive exercise in normocapnic, mild hypercapnic, and severe hypercapnic conditions in human subjects.
Methods
Subjects
Eight healthy, active males with no history of cardiorespiratory diseases volunteered to participate in the present study. Age, height, and weight (mean ± SD) were 23.4 ± 1.8 years, 171.9 ± 6.6cm, and 64.0 ± 6.8kg, respectively. Informed consent was obtained from each subject after a full explanation of the experimental procedure as well as its risks was provided. The experimental protocol was approved by the Human Subjects Committee at the Chukyo University Graduate School of Health Sciences.
Experimental protocol
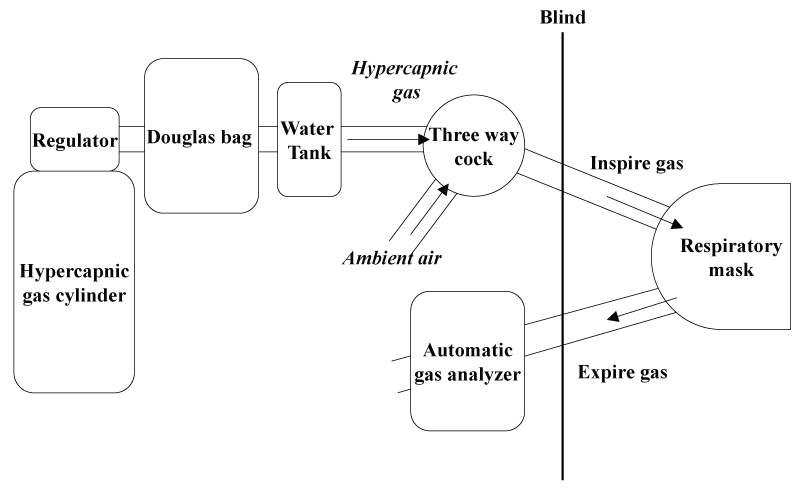
Each subject performed an incremental exhaustive exercise on three occasions in a climatic chamber with fan at a temperature of 26°C, relative humidity of 50%, and normal barometric pressure, under the following conditions: (1) breathing ambient air (Air), (2) breathing mild hypercapnic gas (21% O2, 3% CO2, N2=balance) (3% CO2), and (3) breathing severe hypercapnic gas (21% O2, 6% CO2, N2=balance) (6% CO2). Exercise tests were performed in random order. The interval between each exercise test was at least 1 week. On the day before the exercise test, subjects were advised to avoid strenuous exercise, alcohol, caffeine, smoking, and to fast after supper. Subjects breathed the allocated gas mixture from 10 min before the start of exercise to 5 min after the end of exercise. The subjects were blinded to the inhaled gas composition (Figure 1). Exercise tests were conducted using an electrically-braked cycle ergometer (AEROBIKE75XL; Combi Wellness, Tokyo, Japan); the workload was set at 30 watts (W) at the beginning of the test and increased by 30 W every 2 min until exhaustion. Subjects were encouraged to maintain a pedaling rate of 60 rpm, with exhaustion being defined as the rate falling to less than 40 rpm.
Measurement of cardiorespiratory parameters
During the experiment, expired gas was sampled and oxygen uptake ( ), CO2 output ( ), RER= , expired ventilation ( ), and partial pressure of end tidal CO2 ( ) were analyzed every 30 s using an automatic gas analyzer (RM300, MG360; Minato Medical Science, Osaka, Japan). Heart rate (fH) was also analyzed every 30 s using a heart rate monitor (Life Scope B; Nihon Kohden, Tokyo, Japan). The automatic gas analyzer was calibrated before each test.
Arterialized venous blood analysis
In order to arterialize venous blood, each subject immersed one hand in warm water (40-43°C) for 30 min before gas inhalation, according to Forster et al.'s method [8]. A catheter (22G 1 1/4'') was inserted in a superficial forearm vein and arterialized blood samples were withdrawn through 3-way valves flushed with saline and heparin. Blood samples were collected before exercise, during the last minute of each intensity level, and immediately after exercise. To determine plasma Am levels, 1 ml blood samples were treated with a kit (Ammonia-Test Wako; Wako Company, Osaka, Japan), and the amount of indophenols produced was measured using a spectrophotometer (UV-2400PC; Shimadzu, Tokyo, Japan) at a wavelength of 630 nm. To measure plasma La concentrations, 1 ml blood samples were placed in chilled tubes containing EDTA-2Na and kept in ice water. Whole blood was separated by cold centrifugation (4°C, 1670 × g) and plasma La concentrations were measured enzymatically (2300 Yellow Springs Instrument Co., Yellow Springs, OH, USA). Each subject's AmT and LT were determined from two linear regression lines on the log [Am or La] against log [ ] plot based on log-log transformation [9]. Blood samples for determination of pH, HCO3-, and blood partial pressure of CO2 ( ) levels were drawn into heparinized syringes and promptly measured using a blood gas analyzer (Rapid lab™ 348; Bayer, Germany).
Statistics
Mean values with standard deviation (SD) are presented in tabular and graphical format. As maximal workload differed between the subjects, we unified the data by using as a relative intensity of evaluated pre-exercise: 30%, 45%, 60%, 80%, 90%, and 100% . For statistical comparisons of changes in cardiorespiratory and blood gas parameters as well as plasma Am and La concentration during exercise, a two-way (gas × time) analysis of variance (ANOVA) with repeated measurements was applied. Differences in exercise performance parameters, AmT, and LT among the three tests were analyzed by one-way ANOVA. When a significant effect was found in ANOVA, Fisher's LSD post-hoc test was used to compare means. The statistical package (PASW statistics 18; SPSS, Tokyo, Japan) was used for statistical analysis. Probability values of P<0.05 were considered significant.
Results
Exercise performance
Parameters for exercise performance are summarized in table 1. Performance time was lower for 6% CO2 than Air and 3% CO2 (P< 0.05), while maximal workload was not significantly different among the three conditions.
![]()
Table 1: Exercise parameters due to incremental exhaustive exercise at the three CO2 levels.
View Table 1
Am and La
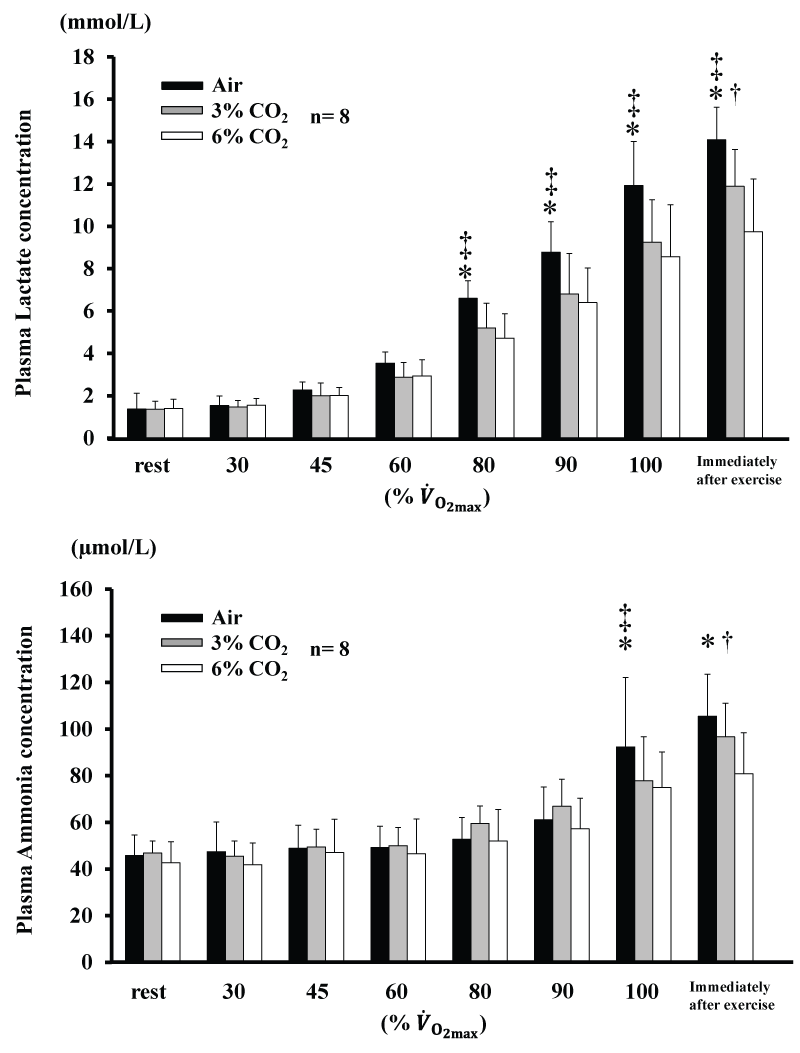
Changes in plasma Am and La concentrations are shown in figure 2. Am for 6% CO2 was lower than Air at 100% and immediately after exercise (P<0.05), and was also lower for 6% CO2 than 3% CO2 immediately after exercise (P<0.05). Am for 3% CO2 was lower than Air at 100% (P<0.05). La for 6% CO2 and 3% CO2 were lower than for Air from 80% (P<0.05), and was lower for 6% CO2 than 3% CO2 immediately after exercise (P<0.05). AmT and LT are shown in table 2. AmT and LT were not significantly different among the three conditions.
.
Figure 2: Changes in concentrations of plasma lactate and plasma ammonia during incremental exercise at the three CO2 levels. Values are means ± SD.
*P<0.05 between Air and 6% CO2. ‡ P<0.05 between Air and 3% CO2. † P<0.05 between 6% CO2. and 3% CO2. .
View Figure 2
![]()
Table 2: Ammonia threshold (AmT) and lactate threshold (LT) at the three CO2 levels
View Table 2
Cardiorespiratory parameters
Gas exchange data are summarized in table 3. For 6% CO2, one subject had a higher and a lower . Consequently, he showed unusually low estimates of RER (0.32-0.4) at 6% CO2 only, which was considered outliers compared to mean estimates for the other subjects (0.52-0.89). Therefore, gas exchange responses for 6% CO2 were reported for the remaining 7 subjects. levels were not significantly different among the three conditions. was lower for 6% and 3% CO2 than Air at strenuous exercise intensity (P<0.05). Moreover, for 6% CO2 was lower than 3% CO2 at 80% (P<0.05). RER for 3% and 6% CO2 were lower than Air throughout the experiment (P<0.05). Further, RER for 6% CO2 was lower than for 3% CO2 at rest and at 30% (P<0.05).
![]()
Table 3: Changes in Oxygen uptake (
), Carbon dioxide output (
), and Respiratory exchange ratio (RER) at rest and during incremental exercise at the three CO2 levels
View Table 3
and fH data are summarized in table 4. for 6% CO2 and 3% CO2 was higher than Air during exercise (P<0.05). Moreover, for 6% CO2 was higher than for 3% CO2 up to and including 60% (P<0.05). fH for 6% CO2 was higher than Air at rest and 45% (P< 0.05), it was also higher than 3% CO2 at 45% (P<0.05).
and
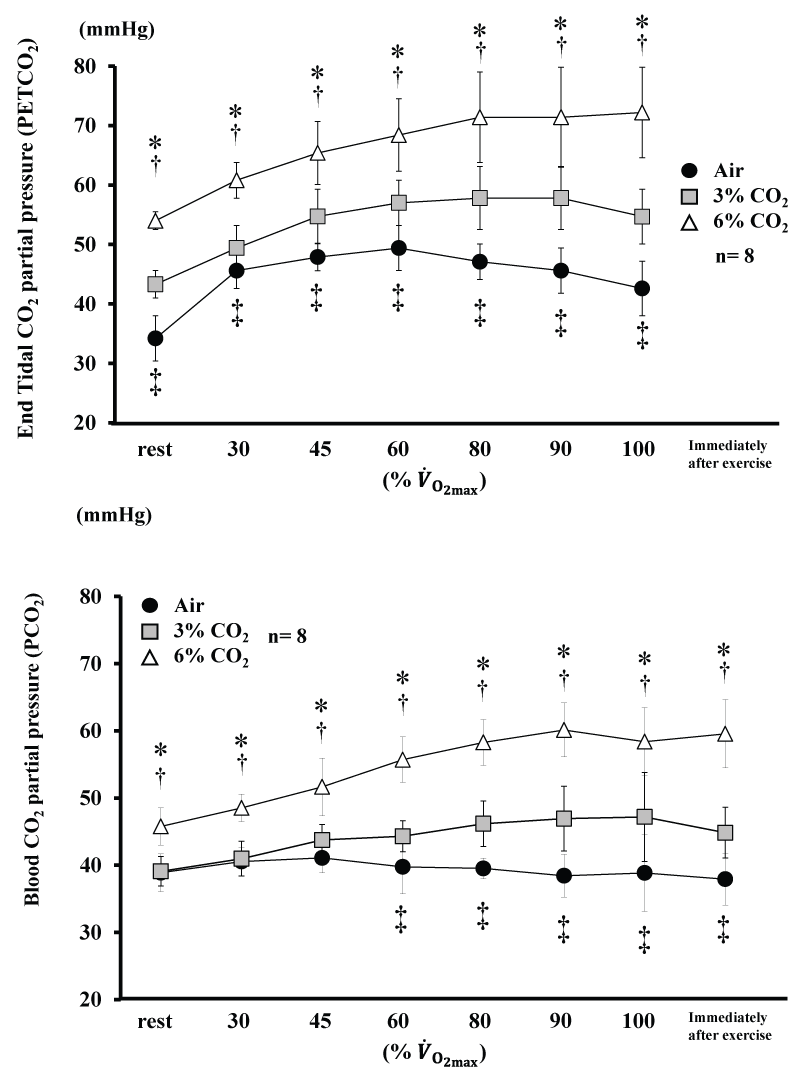
Changes in and blood are shown in figure 3. and blood for 6% CO2 were higher than Air and 3% CO2 throughout the experiment (P<0.05). for 3% CO2 was higher than Air throughout the experiment (P<0.05), and blood for 3% CO2 was higher than Air from 60% (P< 0.05).
.
Figure 3: Changes in end tidal CO2. partial pressure and blood CO2. partial pressure during incremental exercise at the three CO2. levels. Values are means ± SD.
*P<0.05 between Air and 6% CO2. ‡P<0.05 between Air and 3% CO2. †P<0.05 between 6% CO2. and 3% CO2. .
View Figure 3
![]()
Table 4: Changes in Expired ventilation (
) and Heart rate (fH) at rest and during incremental exercise at the three CO2 levels.
View Table 4
Blood pH and HCO3-
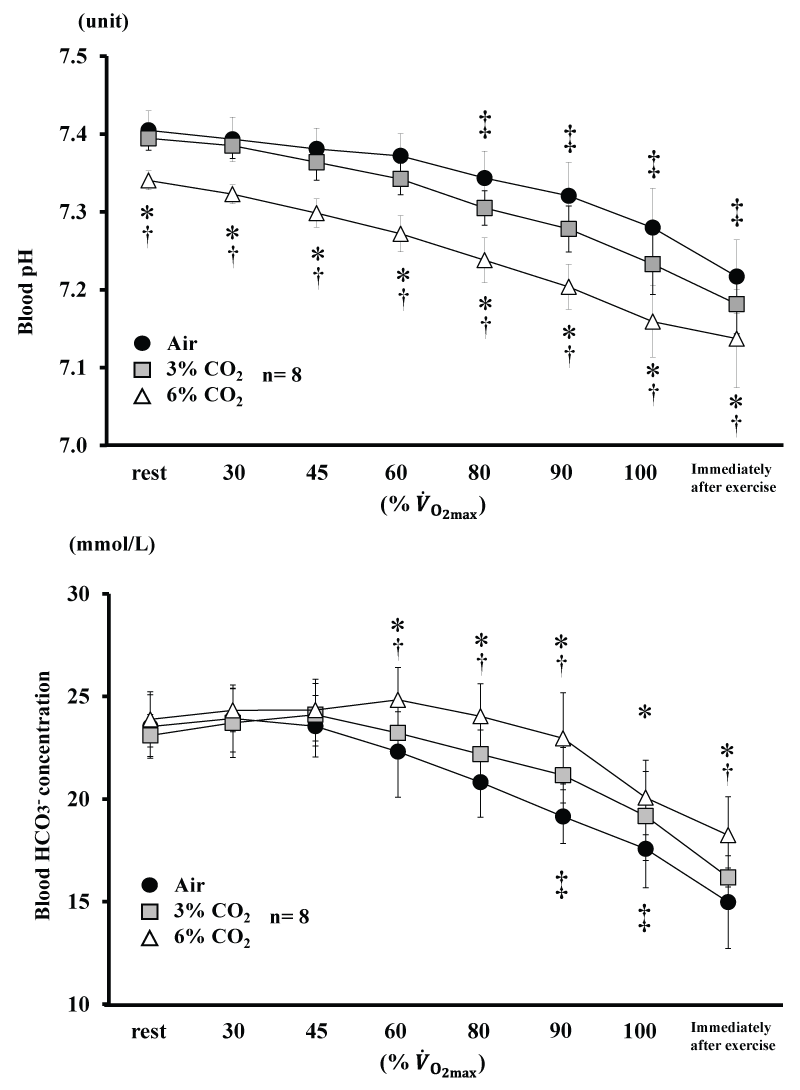
Changes in blood pH and HCO3- are shown in figure 4. Blood pH for 6% CO2 was lower than Air and 3% CO2 throughout the experiment (P<0.05). Blood pH for 3% CO2 was lower than Air from 80% (P<0.05). Blood HCO3- for 6% CO2 was higher than Air and 3% CO2 from 60% (P<0.05). Blood HCO3- for 3% CO2 was higher than Air at 90 and 100% (P<0.05).
.
Figure 4: Changes in blood pH and blood HCO3- during incremental exercise at the three CO2. levels. Values are means ± SD.
*P<0.05 between Air and 6% CO2. ‡P<0.05 between Air and 3% CO2. †P<0.05 between 6% CO2. and 3% CO2.
View Figure 4
Discussion
The primary findings of this study were that: (1) the accumulation of Am due to incremental exercise was inhibited with increasing inhaled CO2 concentration without changing AmT and (2) RER in both hypercapnic conditions were lower compared with Air, but CO2 storage as indicated by a rise in blood for 6% CO2 occurred at rest, while CO2 storage for 3% CO2 was observed from 60% . RER at 30% for 3% CO2 was 0.74 without CO2 storage, which was close to 0.7 (100% fat metabolism). Without CO2 storage steady states can be assumed and an effect on metabolism itself inferred. This is not the case when a constant is not maintained.
Incremental exercise performance under hypercapnia
We previously reported that maximal exercise performance for a 6% inhaled CO2 condition was significantly reduced to approximately 85% of that observed in normocapnia [5]. By contrast, 3% CO2 gas inhalation had a minor effect on maximal exercise workload [10]. Therefore, we adopted 6% CO2 as severe hypercapnia, and 3% CO2 as a mild hypercapnia in this experiment. Since the present results were in accordance with previous results, this made it possible to compare the Am accumulation and the changes in RER with limited exercise performance status and equivalent exercise performance status.
Effect of hypercapnia on ammonia accumulation
AmT was estimated during graded exercise along with LT [11-13]. LT did not change significantly by hypercapnia [4], but the influence of hypercapnia on AmT was unknown. In the present study, we found a reduced Am at 100% , and a reduced La from 80% under both hypercapnic conditions without changing AmT and LT (Figure 2 and Table 2). Therefore, these results suggest that AmT may not be influenced by hypercapnic severity and the suppression of Am accumulation under hypercapnia occurs at exercise intensity above AmT.
The suppression of Am accumulation under hypercapnic conditions may relate to the observed reduced exercise performance. However, compared with Air, there was no difference in exercise performance for 3% CO2 (Table 1), indicating that the lower Am and La were not solely caused by the reduction in incremental exercise performance. A previous study showed that accumulation of La was inhibited as the CO2 concentration of the inhaled gas increased [2]. In the present study, immediately after exhaustive exercise, plasma Am and La concentrations for 6% CO2 were lower compared with those for 3% CO2 and for Air (Figure 2). Moreover, blood pH above 90% decreased with increasing inhaled CO2 concentration (Figure 4). Thus, these results suggest that the lowered Am and La during exercise were caused by the severity of hypercapnia. Previous studies have suggested that lowered blood La might result from the inhibition of phosphofructokinase (PFK) activity or decreased La transport from muscle cells into the blood by hypercapnia-induced respiratory acidosis [14,15]. In a study of skeletal muscle, metabolic acidosis by NH4Cl ingestion in skeletal muscle was reported to decrease La production due to inhibition of glycogenolysis [15]. Thus, systemic acidosis with hypercapnia might inhibit La production. Adenosine monophosphate (AMP) deamination as a source of Am is promoted by lactic acidosis [17]. Furthermore, the activity of PFK is promoted by Am [18,19] and there is a strong relationship between the activity of AMP deaminase and PFK activation [20]. Blood HCO3- is an index of the buffer system [21], and our previous study showed that blood HCO3- was significantly higher for hypercapnia than Air [5]. In support, we found that the reduction in HCO3- during incremental exercise decreased with increasing inhaled CO2 concentration (Figure 4), and this result suggests that lactic acidosis decreased with the severity of hypercapnia. Therefore, it appears that the decrease in accumulation of La during exercise with increasing inhaled CO2 was caused by increasing hypercapnia-induced respiratory acidosis. As a result, a decreased metabolic acidosis by lowered La production might inhibit the accumulation of Am during exercise under hypercapnia.
Effect of hypercapnia on RER and CO2 storage
Concerning decreased RER during hypercapnia, it could be caused by a reduction of due to CO2 storage. RER reflects the pulmonary exchange of and [22], and the present results showed that was not significantly changed by hypercapnia (Table 3). Also, some studies reasoned that the lowered RER during steady state exercise under hypercapnia reflected an actual change in substrate utilization [2,6]. Thus, the application of hypercapnic gas inhalation may be effective to promote fat metabolism during exercise. Generally, respiratory quotient (RQ) for various combinations of fat and carbohydrate (CHO) metabolism is constrained to the values between 0.70 and 1.00 [22]. RER reflects RQ only during rest and steady state exercise, because RER rises above 1.00 in exhaustive exercise or decreases below 0.70 during CO2 storage. In the present study, RER in both hypercapnic conditions showed a lower value than Air and the value of RER for 6% CO2 at rest and 30% was significantly lower than 3% CO2 (Table 3), but it was outside of this range. Moreover, as indicated by blood and for 6% CO2, CO2 storage occurred before exercise (Figure 3). Thus, the application of the 6% CO2 to health science is inappropriate. On the other hand, the mean value of RER at 30% for 3% CO2 was 0.74 (Table 3), which was close to 0.7 (100% fat metabolism). The best exercise training intensity to promote fat metabolism is below 45% intensity as viewed from the "crossover" concept which means that increments in relative exercise intensity result in increasingly greater dependence on CHO and less dependence on fat [7]. Previous studies [2,6], evaluated decreased RER using exercise intensity above 45% .
When increases over 54mmHg, diaphragm fatigue occurs [23]. Also, for endurance cycling load at 80-85% , with 3% CO2 was 51.0 mmHg at end exercise [24]. In comparing the effects of three levels of (low=29mmHg, medium=47mmHg, high=57mmHg), high CO2 tensions significantly impaired cognitive and psychomotor performance at atmospheric pressure [25]. From these previous results, a physiologically and psychologically unusual response may occur when is over 50mmHg. In the present study, value at 30% for 3% CO2 was less than 50mmHg and blood did not significantly change from Air for this condition (Figure 3). Arterialized blood was considered as the best measure of . Concerning the cardiorespiratory responses during the experiment for 3% CO2, was significantly higher, but fH not significantly different compared to Air (Table 4). Therefore, it seems possible that 3% CO2 gas inhalation in combination with low intensity exercise might be advantageous for fat metabolism without CO2 storage and cardiac burden.
Summary
We demonstrated that the accumulation of Am and the mean value of RER due to incremental exercise were reduced with increasing hypercapnia. Above the AmT, the suppression of Am accumulation was observed without changing AmT, while the reduction of steady state RER was indicated at low intensity exercise. When hypercapnic gas inhalation is applied to increase fat metabolism, 3% CO2 inhalation and 30% intensity exercise was the best combination from the viewpoint of RER value and avoiding CO2 storage.
Perspectives
We determined the effect of hypercapnic severity on Am accumulation and RER during incremental exercise. Am accumulation during exercise differs from La accumulation depending on exercise intensity and exercise duration [26,27]. During steady state exercise, plasma glycerol and free fatty acids (FFA) were higher under hypercapnia, but this change did not reach statistical significance [1]. Therefore, future studies are required to determine the accumulation of Am and La during steady state and prolonged submaximal exercise along with indices of fat metabolism. The enhancement of lipid metabolism by a combination of moderate hypercapnic gas inhalation and moderate intensity exercise may be applied to the area of rehabilitation and health sciences. In patients with chronic obstructive pulmonary disease (COPD), exercise performance level was lower in comparison with normal lung subjects [28]. In COPD patients, there were the patients whom Am during exercise rose and the patients whom Am did not rise [29]. Thus, it will be beneficial to adjust exercise intensity and hypercapnic severity to minimize Am response during exercise for therapy.
Acknowledgements
We thank all subjects who participated in this study and the staff of the laboratory for exercise physiology and biomechanics at Chukyo University. This research was supported in part by a Grant-in-Aid for Young Scientists (B) from the Japanese Ministry of Education, Culture, Sports, Science and Technology (Grant No. 18700518 and 20700518).
References
-
Ehrsam RE, Heigenhauser GJ, Jones NL (1982) Effect of respiratory acidosis on metabolism in exercise. J Appl Physiol Respir Environ Exerc Physiol 53: 63-69.
-
Graham TE, Wilson BA, Sample M, Van Dijk J, Goslin B (1982) The effects of hypercapnia on the metabolic response to steady-state exercise. Med Sci Sports Exerc 14: 286-291.
-
McCartney N, Heigenhauser GJ, Jones NL (1983) Effects of pH on maximal power output and fatigue during short-term dynamic exercise. J Appl Physiol Respir Environ Exerc Physiol 55: 225-229.
-
McLellan TM (1991) The influence of a respiratory acidosis on the exercise blood lactate response. Eur J Appl Physiol Occup Physiol 63: 6-11.
-
Kato T, Tsukanaka A, Harada T, Kosaka M, Matsui N (2005) Effect of hypercapnia on changes in blood pH, plasma lactate and ammonia due to exercise. Eur J Appl Physiol 95: 400-408.
-
Ostergaard L, Kjaer K, Jensen K, Gladden LB, Martinussen T, et al. (2012) Increased steady-state VO2 and larger O2 deficit with CO2 inhalation during exercise. Acta Physiol (Oxf) 204: 371-381.
-
Brooks GA, Mercier J (1994) Balance of carbohydrate and lipid utilization during exercise: the "crossover" concept. J Appl Physiol (1985) 76: 2253-2261.
-
Forster HV, Dempsey JA, Thomson J, Vidruk E, DoPico GA (1972) Estimation of arterial PO2, PCO2, pH, and lactate from arterialized venous blood. J Appl Physiol 32: 134-137.
-
Beaver WL, Wasserman K, Whipp BJ (1985) Improved detection of lactate threshold during exercise using a log-log transformation. J Appl Physiol (1985) 59: 1936-1940.
-
Babb TG (1997) Ventilation and respiratory mechanics during exercise in younger subjects breathing CO2 or HeO2. Respir Physiol 109: 15-28.
-
Buono MJ, Clancy TR, Cook JR (1984) Blood lactate and ammonium ion accumulation during graded exercise in humans. J Appl Physiol Respir Environ Exerc Physiol 57: 135-139.
-
Yuan Y, So R, Wong S, Chan KM (2002) Ammonia threshold--comparison to lactate threshold, correlation to other physiological parameters and response to training. Scand J Med Sci Sports 12: 358-364.
-
Yuan Y, Chan KM (2004) A longitudinal study on the ammonia threshold in junior cyclists. Br J Sports Med 38: 115-119.
-
Graham TE, Barclay JK, Wilson BA (1986) Skeletal muscle lactate release and glycolytic intermediates during hypercapnia. J Appl Physiol (1985) 60: 568-575.
-
Spriet LL, Matsos CG, Peters SJ, Heigenhauser GJ, Jones NL (1985) Effects of acidosis on rat muscle metabolism and performance during heavy exercise. Am J Physiol 248: C337-347.
-
Hollidge-Horvat MG, Parolin ML, Wong D, Jones NL, Heigenhauser GJ (1999) Effect of induced metabolic acidosis on human skeletal muscle metabolism during exercise. Am J Physiol 277: E647-658.
-
Hellsten Y, Richter EA, Kiens B, Bangsbo J (1999) AMP deamination and purine exchange in human skeletal muscle during and after intense exercise. J Physiol 520: 909-920.
-
Mutch BJ, Banister EW (1983) Ammonia metabolism in exercise and fatigue: a review. Med Sci Sports Exerc 15: 41-50.
-
Sugden PH, Newsholme EA (1975) The effects of ammonium, inorganic phosphate and potassium ions on the activity of phosphofructokinases from muscle and nervous tissues of vertebrates and invertebrates. Biochem J 150: 113-122.
-
Norman B, Hellsten-Westing Y, Sjödin B, Jansson E (1994) AMP deaminase in skeletal muscle of healthy males quantitatively determined by new assay. Acta Physiol Scand 150: 397-403.
-
Péronnet F, Aguilaniu B (2006) Lactic acid buffering, nonmetabolic CO2 and exercise hyperventilation: a critical reappraisal. Respir Physiol Neurobiol 150: 4-18.
-
McArdle WD, Katch FI, Katch VL (2007) Exercise physiology: Energy, Nutrition, and Human Performance, 6th edition. Lipincott Willams & Wilkins, Philadelphia, PA: 189-194.
-
Juan G, Calverley P, Talamo C, Schnader J, Roussos C (1984) Effect of carbon dioxide on diaphragmatic function in human beings. N Engl J Med 310: 874-879.
-
Shykoff BE, Warkander DE (2012) Exercise carbon dioxide (CO2) retention with inhaled CO2 and breathing resistance. Undersea Hyperb Med 39: 815-828.
-
Fothergill DM, Hedges D, Morrison JB (1991) Effects of CO2 and N2 partial pressures on cognitive and psychomotor performance. Undersea Biomed Res 18: 1-19.
-
Ogino K, Kinugawa T, Osaki S, Kato M, Endoh A, et al. (2000) Ammonia response to constant exercise: differences to the lactate response. Clin Exp Pharmacol Physiol 27: 612-617.
-
Urhausen A, Kindermann W (1992) Blood ammonia and lactate concentrations during endurance exercise of differing intensities. Eur J Appl Physiol Occup Physiol 65: 209-214.
-
Castagna O, Boussuges A, Vallier JM, Prefaut C, Brisswalter J (2007) Is impairment similar between arm and leg cranking exercise in COPD patients? Respir Med 101: 547-553.
-
Calvert LD, Singh SJ, Greenhaff PL, Morgan MD, Steiner MC (2008) The plasma ammonia response to cycle exercise in COPD. Eur Respir J 31: 751-758.