AECOPD have major implications on the quality of life, morbidity and mortality of COPD patients. In addition to their assessment on clinical presentation, which can be variable and difficult to predict, a large number of biomarkers are used. Inflammation increases during exacerbations of COPD and there are changes in systemic markers like CRP, IL 6 and PARC/CC18, as well as the cell structure in sputum and blood. The aim of this study was to investigate the diagnostic and prognostic value of plasma biomarkers levels, sputum and hematic cell profile in patients with AECOPD. Mean concentration of serum markers studied were higher in first consultation, and significantly decreasing 21 days after. The number of cells in sputum and their structure, number of blood leucocytes has significant differences with results after 21 days. NLR resulted a reliable indicator and simple in the determining of inflammation growth. Diagnosis of AECOPD is supported by increased sputum inflammation and increased systemic inflammation as demonstrated by increased number of blood cells. IL-6, PARC/CCL-18, and CRP resulted useful for diagnosis of AECOPD and to follow-up stabilization. AECOPD inflammation is more evident in stage IV of the diseases.
Seric marker, CRP, IL6, PARC/CCL18, AECOPD
The reporting of symptoms by patients and interpretation by the physicians can be prone to subjective view, which makes the finding of more objective criteria for the disease activity, necessary. So, the use of lab parameters to improve the diagnostic accuracy of acute exacerbation of chronic obstructive pulmonary disease (AECOPD) is a field of interest where studies are being conducted in continuity.
The study of the diagnostic value of systemic markers like CRP, IL 6 and PARC/CC18, as well as the cell structure in sputum and blood, in the AECOPD.
The abstract is a prospective study. 56 patients with AECOPD have been studied, 54 (96%) being male, 29 (52%) in stage III and 27 (48%) in stage IV. Anamnestic, clinical and lab data (CRP, IL 6, PARC/CCL18, cell content of sputum and blood) have been evaluated at first consultation, and after 21 days of AECOPD. Characteristics of the patients in the study are demonstrated in Table 1.
Table 1: Characteristics data of patients. View Table 1
For determining CRP the latex immune turbidimetric method of working is used. PCR measurements have been conducted through the Cobas c 111 instrument, using fotometric analysis and an optional unit for the ion selective electroce (ISE). In evaluating CRP levels, cut off for normal: 5-10, light inflamation: 10-40, active bacterial inflamation: 40-200, severe bacterial > 200 mg/l is used.
Determining Interleukin-6 (IL-6) in serum is used with the immunoassay with electrochemiluminescence "ECLIA" used in Elecsys and Cobas immonoassay analyzer. For evaluating marker IL6 level, cut off 7 pg/ml is used.
Determining PARC/CCL18 is done via the ELISA kit of CCL18 (human), which is an immunosorbent enzyme-linked in vitro test, for meassuring correct human PARC, in serum, plasma and supernatant cell culture. The testing is conducted by using the quantitative sandwich enzyme immunoassay technique.
Hematological examination was conducted with Sysmex XS-100i Automated Hematology Analyzer, via fluorescent cytometry.
Examination of sputum has been conducted with the material that has been expectorated early in the morning after a deep inhale followed by rough coughing. Sputum has been stratified immediately in lames, fixed with 95% ethanol, dried and then colored via the Papanikolau coloration. Then the sputum smear is examined under the microscope. If there are macrophages in sufficient quantity, it is clear that we have to do with sputum, not saliva. If the celluar elements dominating are squamose cells, candids or even ciliary epithelial cells, we have to do with elements of the oral or sinonasal apparatus. Depending on the dominance of each of the present cellular elements in the smears, based on the normal percentage table of their concentrations as well, the classification of each percentage of the cellular elements present in the smear is done. After the percentage of the contents of the cellular elements in the sputum, the cellular structure is classified as neutrophilic, eosinophilic or paucigranulocitic.
All of the data collected was mapped onto Microsoft Excel, from where they were exported in në SPSS (Statistical Package for Social Sciences) 20.0 and Medstat, with which statistical analysis of the data was conducted. The values of P ≤ 0.05 were considered significant.
AECOPD has increased significantly of total sputum cells in 39 (69.6%), macrophage -55 (98.2%), neutrophils -51 (91.1%), lymphocytes -37 (66.1%), eosinophils -19 (33.9%) and epithelial cells -9 (16.1%).
After 21 days of treatment have remained increased total cells in 13 (23.2%), neutrophils -13 (23.2%), lymphocytes -45 (80%), eosinophils -6 (10.7%), epithelial cells -26 (46.1%), and totally normal or decreased macrophages.
The number of cells in sputum and their structure expressed in percentage in AECOPD has significant differences with results after 21 days (P < 0.0001) (Table 2).
Table 2: Sputum cell comparison at AECOPD and 21 days after. View Table 2
All of the AECOPD patients were stratified, according to the number of neutrophils (> 61%) and eosinophils (> 2.5%) in the sputum samples. Individual patients were classified into the eosinophilic (EO) with sputum eosinophils > 2.5% of total cells, the neutrophilic (NE) with neutrophils > 61%, the paucigranulocytic COPD (PA) with eosinophils ≤ 2.5% and neutrophils ≤ 61%. Cell sputum stratification resulted: eosinophilic 9 (16.1%), neutrophilic 16 (28.6%) and paucigranulocytic 31 (55.4%) (Table 3).
Table 3: Stratification according to cell content in sputum. View Table 3
There were sinjificant differences in number of cells and percent of neutrophils in sputum at AECOPD according to the stage of disease (Table 4).
Table 4: Sputum cell comparison at AECOPD according to the stage of diseases. View Table 4
The average initial blood leukocytosis was 11777 ± 5233, after 21 days in 2630 ± 8593 (P < 0.0001). AECOPD leukocyte formula (%) and after 21 days resulted respectively: rod nuclear 6.63 ± 3.68 and 2.79 ± 2.51 (P < 0.0001), neutrophils 12.38 ± 72.41 and 60.68 ± 10.12 (P < 0.0001), eosinophils 2.1 ± 2.69 and 3.81 ± 3.49 (P = 0.0045), basophils 0.21 ± 0.27 and 0.22 ± 0.28 (P = 0.8478), monocytes 8.15 ± 4.53 and 7.49 ± 3.15 (P = 0.3727), lymphocytes 17.07 ± 8.80 and 27.62 ± 8.19 (P < 0.0001) (Table 5).
Table 5: Leukocytic formula at AECOPD and 21 days after. View Table 5
There was increased level of leukocytes in 35 (62.5%) patients, rod nuclear 26 (46.4%), neutrophils 28 (50%), eosinophils 7 (12.5), basophils 1 (1.8%), monocytes 21 (37.5%), and lymphocytes 1 (1.8%).
Whereas after 21 days, the leucocyte level has remained increased in 5 (8.9%), the stick - 1 (1.8%), eosinophilic - 13 (23%), neutrophilic and basophilic - none, monocytes - 16 (28.6%) and lymphocytes - 3 (5.4%).
NLR (report neutrophil/lymphocytes) at first consultation and after 21 days have respectively resulted 7.464 ± 12.922 (1.04 - 97.9) and 2.509 ± 1.18 (0.71 - 7.09) (P = 0.004).
From the results of the haemogram analysis in patients with AECOPD, stage III and IV, significant differences in leukocyte numbers were noticed, whereas in cellular structure (expressed in %), there are significant differences between the two stages in the lymfocytes. In the comparison between two groups of disease gravity with the cellular structure groups, there are significant differences (Table 6).
Table 6: Leukocytic formula at AECOPD according to the stage of diseases. View Table 6
Mean concentration of serum markers studied were higher in first consultation of subjects for AECOPD and significantly decreasing after 21 days (P < 0.0001) respectively: IL60 33.46 pg/ml ± 45.9 (min 3.1 max 232.3), IL621 9.1 pg/ml ± 7.5 (min 1.7 max 43.6); PARC/CCL-180 77 ng/ml ± 34.5 (min 24.6 max 168.8), PARC/CCL-1821 51.2 ng/ml ± 23.5 (min 10.7 max 107.2); CRP0 43.1 mg/l ± 49.2 (min 0.2 max 257), CRP21 11.2 mg/l ± 12 (min 0.1 max 48). There were no correlations among these biomarkers with clinical variables (Figure 1).
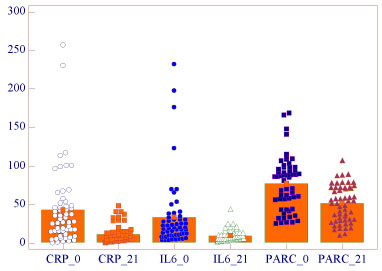 Figure 1: Biomarkers at AECPOD and 21 days after. View Figure 1
Figure 1: Biomarkers at AECPOD and 21 days after. View Figure 1
Levels of CRP, IL6, PARC/CCL18 (cut off 30 and 60 ng/ml) at AECOPD and 21 days after are seen in Figure 2, Figure 3, Figure 4 and Figure 5, respectively.
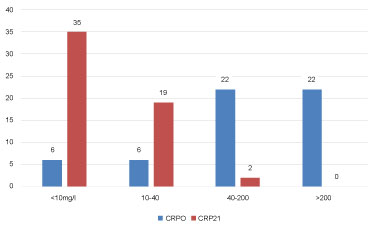 Figure 2: CRP levels at AECOPD and 21 days after. View Figure 2
Figure 2: CRP levels at AECOPD and 21 days after. View Figure 2
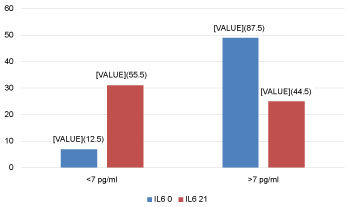 Figure 3: IL6 levels at EACOPD and 21 days after (%). View Figure 3
Figure 3: IL6 levels at EACOPD and 21 days after (%). View Figure 3
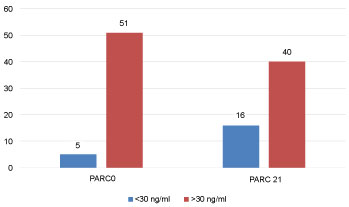 Figure 4: PARC/CCL18 levels at EACOPD and 21 days after (%). View Figure 4
Figure 4: PARC/CCL18 levels at EACOPD and 21 days after (%). View Figure 4
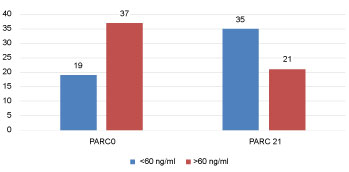 Figure 5: PARC/CCL18 levels at EACOPD and 21 days after (%). View Figure 5
Figure 5: PARC/CCL18 levels at EACOPD and 21 days after (%). View Figure 5
Levels of CRP, IL6, PARC/CCL18 (cut off 30 and 60 ng/ml) at AECOPD according to the category of COPD are seen in Table 7, Table 8, Table 9 and Table 10, respectively.
Table 7: CRP levels at AECOPD according to the category of COPD. View Table 7
Table 8: IL6 levels at AECOPD according to the category of COPD. View Table 8
Table 9: PARC levels at AECOPD according to the category of COPD. View Table 9
Table 10: PARC levels at AECOPD according to the category of COPD. View Table 10
Studies of bronchial inflammation of COPD patients have provided contradictory results. A study with bronchial biopsy resulted in a 30 fold increase of eosinophils, small increases in neutrophils and T-lymphocytes [1], whereas in another study with bronchoalveolar lavage, liquid resulted with the neutrophils and eosinophils, but with a more pronounced increase in the neutrophils [2]. Noninvasive studies of sputum are more easily conducted, but then again results are contradictory: Without changes in cell count [3] or increase in lymphocytes, neutrophils and eosinophils [4]. According to authors, neutrophils results to be connected with the gravity of exacerbations independent of etiology, whereas eosinophilia as an indicator of viral exacerbations [5].
In our study it results that in 33.9% of cases, there has been an increase of eosinophils and according to the stratification of sputum the eosinophilic type has resulted in 16.1% of the patients. The main presentation during exacerbation of bronchial secretion is increased neutrophils [2], which resulted even in our study, where the cellular profile of sputum together with the neutrophilic (28.6%) and paucigranulocitic (55.4%) results in 84% of the cases, which is connected even with the appearance of change of purulence in sputum [6].
From our data, after 21 days of exacerbation, in the sputum there is a decrease in the number of total cells, a decrease in the neutrophils and eosinophils percentage, as well as an increase in percentage of lymphocytes. The cell count in sputum as well as their structure expressed in percentage in AECOPD has significant differences with the results after 21 days (P < 0.0001). As it results even from our data, at the time of the exacerbation remission, there is a decrease in the number of neutrophils, which is related to the eradication of the bacteria from the sputum [7]. During a COPD exacerbation, great observing studies haven't shown a significant increase of macrophages in sputum, or the bronchial tissues, even as a percentage of the total cell and neither as an absolute increment of cells [3,5]. In our study the result is different - an increase of macrophages in the exacerbation.
There has been a significant difference in the average number of blood leucocytes in AECOPD and 21 days after (P < 0.0001). Just so, the leucocytary formula (%) in AECOPD and after 21 days results in a significant drop of rod nuclear (P < 0.0001), neutrophils (P < 0.0001), an increase of eosinophils (P = 0.0045) and of lymphocytes (P < 0.0001). With the improvement of the exacerbations there is a significant decrease of the leucocyte numbers, mainly as a consequence of the neutrophils dropping. The number of neutrophils in blood increases with the systemic inflammation. The increased number of neutrophils is connected to the progression of COPD [8]. Our data match the ones from the studies where the leucocyte levels have resulted with significant statistically increase in the patients with COPD exacerbations, compared to the ones in remission.
Our results suggest that matching the literature, the NLR can be considered like a reliable indicator and simple in the determining of inflammation growth in patients with COPD. Furthermore, NLR can be useful for discovering early acute exacerbations that are possible in COPD patients [9].
The level of biomarkers studied in the AECOPD, and after 21 days of treatment has resulted in a significant difference which shows their value in exacerbation evaluation of COPD. The levels of markers resulted higher at the first consultation of subjects for AECOPD, and there is a notable decrease after 21 days (P < 0.0001).
CRP is the most well known marker. High levels of systemic CRP have been found connected to the increase of disease gravity, worsening of health condition, hospitalization and mortality in COPD [10]. CRP was the first biomarker to be studied in COPD. Most of the studies have indicated that CRP levels are increased in these patients [11]. A study showed that CRP levels testing in patients with lower respiratory system infections in primary care has reduced antimicrobial prescriptions considerable, without compromising the recovery of the patient [12]. From literature, CRP testing was insignificantly sensible and neither was it specific enough to distinguish an infiltrate in thoracic radiography, and the bacterial etiology of the infection of the lower respiratory tract [13]. In the biomarkers field, the measuring of the procalcitonine levels looks promising as a tool to identify patients with COPD exacerbations, which demand antimicrobial treatment [14].
Levels of IL6 in exacerbations and after 21 days in relation to the value over cut off - 7 pg/ml, resulted with significant difference, which speaks of the value of application in the discovery of exacerbation and the follow-up the inflammation in patients with AECOPD.
PARC/CCL-18 in AECOPD has a significant difference with that after 21 days. There has been research conducted: "Lung Health Study (LHS)" for PARC/CCL-18 in COPD, where different populations of COPD are included, 4800 subjects with light or moderate COPD, 1800 subjects of COPD from the ECLIPSE study, which represent all the GOLD stages, 312 smokers and 226 non-smoking controls, and 89 COPD subjects with prednisolone treatment [15]. Results were somewhat contradictory.
In the LHS, high levels of PARC/CCL-18 were connected with lower FEV1, increased cardiovascular mortality rate. In ECLIPSE study, levels of PARC/CCL-18 were higher on individuals with COPD than in the control group, but there was no correlation with FEV1.
According to cell stratification in sputum, there results that in the exacerbations, with the increase of CRP levels, there is an increase in the neutrophilic and paucigranulocitic groups. In the examination of sputum after 21 days, it is noted that, in relation to the drop in CRP levels, there is a drop of the neutrophilic group, and paucigranulocitic. In the measurements blood level of IL6, classified in value < 7 pg/ml and as an increased level, values > 7 pg/ml, results that in sputum there is a larger neutrophilic and paucigranulocitic in the patients with IL6 > 7 pg/ml. After 21 days, it results that the decrease of IL6 levels is accompanied with a change of cellular structures, with a decrease of neutrophilic and paucigranulocitic groupings. In the definition of PARC, as in cut off 30 ng/ml and 60 ng/ml there is a correlation between the increases of marker levels with the paucigranulocitic group. Parameters such as the level of serial CRP, value of ESR, number of leucocytes and predomination of neutrophils in the leucocitary formula, are used quite often to follow the infection in clinical practice. Different marker levels as in the CRP inflammation, fibrinogen and leucocyte counts are increased in patients with COPD in the period of exacerbations [16].
Liang, et al., studied correlation between changed in CRP levels and resolution of bronchial inflammatory markers, and of clinical health state during the period of recovery, after acute COPD exacerbations. The relation between changes in bronchial inflammatory markers, the CAT and CRP results during the recovery period was studied. Serial levels of CRP in the acceptance of patients in hospitals with negative prognosis have been higher than the ones without it. In comparison with patients without cardiovascular complications, patients with them had higher serial levels of CRP on acceptance. The drop of CRP levels correlated positively with the decrease of neutrophils in sputum in the fourth and seventh days. There has been a significant correlation between drop of CRP and CAT. Thus, the authors reach the conclusion that CRP could be useful in monitoring the resolution of bronchial inflammation and improvement of health state during the treatment of COPD exacerbations [17].
COPD exacerbations caused by bacterial infections have an increase of neutrophils in sputum, often causing a systemic inflammatory reaction: Inflammatory markers such as the neutrophil blood count, CRP, fibrinogen and IL6 in serum, are increased during the exacerbations [18]. There have been several mechanisms, proposed as the origin of systemic inflammation increase. These include: 1) Expression of inflammatory mediators from pulmonary structures; 2) Inflammatory reaction in tissue hypoxia; 3) Reaction, induced by pro-inflammatory bacterial lipopolysaccharide products [19].
From the haemogram analysis results in stage III and IV AECOPD patients, there can be seen significant differences in leucocyte numbers, whereas in cellular structures (expressed in %), there are significant changes between the two stages of lymphocytes. In comparison between the two disease gravity groups, according to the groups of cellular structure, resulting in significant changes. If we compare inflammation intensity (after the grouping of CRP values) initialy and after 21 days in exacerbated COPD of stages III and IV, the inflammation results to have been more pronounced in stage IV and in both periods. In comparison with the initialy IL6 values and after 21 days in stage III and IV of exacerbated CPD, there appear to be higher level in stage IV and both periods, but the difference is more pronounced in the measuring after 21 days. According to the COPD stages, values of initialy PARC are more pronounced in stage IV, more evident for cut off 60. In relation to initialy CRP in normal values, there are no cathegory D4 cases and greater values of the level from 40 to over 200 mg/l are in relations to cathegories D3 and D4. In the results of examinations after 21 days, it is noted that cases are collected in CRP levels below 40 mg/l.
Diagnosis of AECOPD is supported by increased sputum inflammation as proxy of airways inflammation and increased systemic inflammation as demonstrated by increased number of blood cells. IL-6, PARC/CCL-18, and CRP resulted useful for diagnosis of ECOPD and to follow-up stabilization. AECOPD inflammation is more evident in stage IV of the diseases.