Exercise limitation associated with most lung diseases is multifactorial and is due to complex interactions between impaired ventilatory, cardiovascular, and peripheral muscle responses. Cardiopulmonary exercise tests (CPETs) are often required to ascertain the primary cause of exercise limitation particularly in patients with multiple co-morbidities. CPETs are generally offered only at tertiary care medical centers.
To evaluate if forced expiratory flow from 25% to 75% of vital capacity (FEF25-75%) can be used to predict both reduced exercise capacity and ventilatory limitation to exercise.
We retrospectively reviewed paired CPETs and spirometry tests performed on all adult patients (> 18 years of age) in the Pulmonary Physiology Laboratory at St. Elizabeth's Medical Center between April, 2006 and April, 2016 to explore the association between spirometric parameters and ventilatory limitation to exercise. Ventilatory limitation was defined as ventilatory reserve ≤ 15% at peak exercise. We defined reduced exercise capacity as peak oxygen consumption VO(2) < 20 mL/kg/min.
FEF25-75% was strongly associated with low breathing reserve (area under the ROC curve - 0.81). FEF25-75% was also significantly associated with the combined outcome of low ventilatory reserve and reduced exercise capacity. The area under the ROC curve was 0.86 (p < 0.0001) suggesting excellent predictive ability. In patients with FEV25-75% ≥ 40%, exercise limitation was much more commonly non-ventilatory in nature. In patients with mid-expiratory flow, FEV25-75% ≤ 20%, nearly 7 of 10 patients had ventilatory limitations to exercise.
Mid-expiratory flow FEF 25-75% is closely associated with both pulmonary ventilatory limitation to exercise and reduced exercise capacity.
Cardiopulmonary exercise test, Exercise capacity, Pulmonary function tests, Spirometry
Patients with advanced lung disease commonly have reduced exercise capacity [1-4]. Exercise limitation and exertional dyspnea associated with most lung diseases is multifactorial and is due to complex interactions between impaired ventilatory, cardiovascular, and peripheral muscle responses [5-12]. Patients with advanced pulmonary disease also frequently have coexisting cardiovascular diseases, psychological conditions and metabolic disorders which independently impact exercise tolerance. The accurate identification of physiologic limitations to exercise is a critical step in the development of effective therapeutic strategies designed to improve exercise performance and quality of life [8].
Cardiopulmonary exercise tests (CPET) provide a comprehensive and dynamic measurement of integrative exercise responses involving the pulmonary, cardiovascular, hematopoietic, neuropsychological, and skeletal muscle systems which is difficult to accurately assess through individual testing of these organ systems [13]. CPETs are often required to ascertain the primary cause of exercise limitation particularly in patients with multiple co-morbidities. However, CPETs are not readily available and generally offered only at tertiary care medical centers.
Although many factors contribute to exercise intolerance in patients with lung disease, a dominant reason is pulmonary ventilation limitation [12,14]. This limitation may be amenable to therapies like bronchodilator agents resulting in improved exercise capacity. However, in the setting of abnormal baseline lung function determining whether or not a patient's pulmonary ventilatory deficiency is a dominant explanation of exercise intolerance and dyspnea can often be challenging.
Many studies have looked into exercise limitation in chronic obstructive pulmonary disease (COPD) patients. It is well accepted that as COPD progresses; patients experience worsening exercise capacity. A few small studies have demonstrated a significant correlation between resting pulmonary function test (PFT) parameters and overall exercise capacity in COPD patients. These studies however did not explore whether the exercise limitations observed were specifically related to subjects' underlying lung disease and compromised ventilatory capacity as opposed to concurrent co-morbidities [15-19].
Spirometry is the most commonly performed pulmonary function test. Most patients can easily perform spirometry when overseen by a trained health care provider. Spirometry is inexpensive and can be administered in the ambulatory setting, emergency department or inpatient setting. Pinto-Plata, et al. demonstrated a significant reduction in exercise capacity in COPD patients with declining post bronchodilator spirometry measurements [19].
In a population of patients with and without pulmonary disease, we evaluated whether forced expiratory flow from 25% to 75% of vital capacity (FEF25-75%) measured during routine spirometry measurements can be used to predict both reduced exercise capacity and ventilatory limitation to exercise. We think that FEF 25-75% or mid-expiratory flow may be the best spirometric indicator of ventilatory compromise in pulmonary disease.
We retrospectively reviewed paired CPETs and spirometry tests performed on all adult patients (> 18 years of age) in the Pulmonary Physiology Laboratory at St. Elizabeth's Medical Center between April, 2006 and April, 2016 to explore the association between spirometric parameters and ventilatory limitation to exercise.
Patients performed spirometry according to American Thoracic Society (ATS)/European Respiratory Society (ERS) guidelines [20]. Technical procedure acceptability and reproducibility criteria were those recommended by ATS [20]. Percentage predicted values for spirometry data were calculated based on age, sex, height and ethnicity using the predicted normal values for spirometry from the Third National Health and Nutrition Examination Survey, NHANES III reference values [21].
CPETs were performed following ATS/American College of Chest Physician (ACCP) standards [13,14]. Symptom-limited, ramp-incremental cycle ergometer exercise tests were performed on a digital computer-based exercise system and gas exchange was measured using a metabolic cart (V Max; SensorMedics; Yorba Linda, CA). The exercise protocol involved an initial 2 minutes of rest, followed by unloaded cycling for another 2 minutes with an increment every minute of 5-15 watts. Peak VO2 was expressed as absolute value in mL/kg/min.
We excluded CPETs with submaximal exercise or technical issues limiting their interpretation. All spirometry tests were performed within 1 week of the CPET.
To determine ventilatory reserve during CPETs, we calculated maximal voluntary ventilation (MVV) by multiplying FEV1 by 40. MVVs estimated by this method have been shown to be not discernibly different from MVVs directly measured [22,23]. Ventilatory reserve (breathing reserve) was calculated as the difference between MVV and the peak minute ventilation achieved during exercise (VEmax) [24].
Ventilatory reserve (VR) % = 100 (MVV-VEmax)/MVV
Ventilatory limitation was defined as ventilatory reserve less than or equal to 15% at peak exercise.
We defined reduced exercise capacity as peak oxygen consumption VO(2) of < 20 mL/kg/min.
Predictive models were created for two different outcomes: ventilatory reserve ≤ 15% and the combined outcome of ventilatory reserve ≤ 15% with peak V02 < 20 mL/kg/min. Percentage predicted values for forced expiratory flow from 25% to 75% of vital capacity (FEF25-75%) and forced vital capacity (FVC) were identified a priori as candidate variables for each model. FEF 25-75% performed more accurately on initial testing and was ultimately the chosen variable. The area under the ROC curve was calculated to assess discriminative ability of the models. Model diagnostics were checked to identify potential influential points. SAS EG 9.4 was used for all analyses.
345 patients were included in the analysis between the ages of 18 and 87 years (average age of 59.2 years). 147 female and 198 male patients were included in the analysis. Out of the 345 patients included in the analysis, a total of 76 patients (22%) had ventilatory limitation at peak exercise; while 52 of these 76 patients had evidence of both ventilatory limitation and reduced exercise capacity (Table 1).
Table 1: Descriptive data (all values mean ± standard deviation unless otherwise indicated) of the study population undergoing CPET including anthropometrics, spirometry and CPET measurements. BMI-body mass index, VO2-peak oxygen consumption (ml/kg/min), FEV1%-Percent predicted Forced expiratory volume in first second, FVC%-Percent predicted Forced Vital Capacity, FEF25-75%-Percent predicted Forced midexpiratory flow, PEF%-Percent predicted Peak expiratory flow, VE/VCO2- minute ventilation/carbon dioxide production. View Table 1
FEF25-75% was strongly associated with low breathing reserve and performed more accurately than FVC. The area under the ROC curve of 0.81 supports that FEF25-75% measurement has a good predictive ability to identify patients with or without a low breathing reserve (Figure 1).
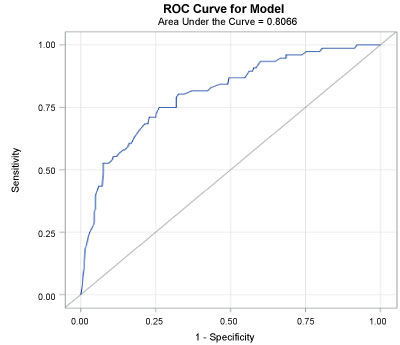 Figure 1: Area under the ROC curve for the breathing reserve with FEV25-75% is 0.81 indicatng FEV25-75% has good ability to identify patients with ventilatory limitation at peak exercise. View Figure 1
Figure 1: Area under the ROC curve for the breathing reserve with FEV25-75% is 0.81 indicatng FEV25-75% has good ability to identify patients with ventilatory limitation at peak exercise. View Figure 1
The positive predictive value of FEV25-75% > 40% for adequate ventilatory reserve at peak exercise (VR > 15%) in our patient population is 88%, meaning nearly 9 in 10 patients with FEV25-75% ≥ 40% did not demonstrate a ventilatory limitation to exercise. Conversely, the positive predictive value of FEV25-75% ≤ 20% for VR < 15% (at peak exercise) in our patient population was 68%, meaning nearly 7 in 10 patients with FEV25-75% ≤ 20% demonstrated a ventilatory limitation to exercise.
FEF25-75% was also significantly associated with the combined outcome of low ventilatory reserve and reduced exercise capacity. The area under the ROC curve was 0.86 (p < 0.0001) suggesting excellent predictive ability.
The probability of the combination of low ventilatory reserve and reduced exercise capacity (CO) can be calculated using FEF 25-75% with the following formula:
1 - {1/[exp((-0.0547 × FEV25/75%) + 0.9315)]}
Determining the cause of dyspnea and reduced exercise capacity is often challenging. While knowing whether a patient has reduced exercise capacity is important, the more relevant clinical challenge is to determine what physiological limitations to exercise are most responsible for an individual patient with the ultimate goal of leveraging these discoveries to develop therapeutic strategies intended to improve exercise performance and quality of life. CPETs are designed to examine primary causes of dyspnea and exercise limitation particularly in patients with multiple co-morbidities. The limited availability of CPETs however presents challenges to those that do not receive their care at tertiary care medical centers.
An ability to parse more out of pulmonary function results to solve this common clinical question is an attractive method to further utilize routinely obtained clinical data. A few studies have examined the correlation between exercise capacity and pulmonary function parameters, though none have directly explored the relationship between spirometry parameters and ventilatory limitation to exercise.
Efremidis, et al. examined the association of exercise capacity with measurements of resting pulmonary function in a total of 57 patients with COPD. FEV1 and FVC had significant (p < 0.001) associations with peak exercise capacity VO2 (area under curve of 0.62 and 0.71 respectively) [15]. Zhang and co-workers investigated the association of post-bronchodilator PFTs with exercise capacity in a cohort of 50 COPD subjects [17]. The area under the curve for FEV1% as predictor of peak VO2 was 0.853 (95% CI 0.751 - 0.955, p < 0.001) [17]. Similarly, Albuquerque, et al. studied exercise capacity in 44 patients with moderately severe COPD and found that the area under the receiver operating characteristic (ROC) curve was 0.86 (0.73 - 0.99) for FEV1 [18]. Lastly, Pinto-Plata, et al. studied 453 COPD patients and noted a significant reduction in exercise capacity for patients from GOLD stage 1 to stage 4 (stage 1 peak VO2 17 ± 5 compared to stage 4 peak VO2 9 ± 3 ml/kg/min) [19].
A limitation to most of these studies has been small sample size. Most of these studies also only included patients with COPD and excluded patients with other pulmonary diseases. Additionally, some limited enrollment further to patients with moderately severe COPD and excluded those with more advanced or milder COPD. These studies also excluded COPD patients with other severe systemic diseases that impair pulmonary function and/or reduce exercise capacity. All these factors present challenges to utilizing those results in real-world clinical settings.
We explored whether spirometry results can predict a ventilatory limitation to exercise in a heterogeneous population of patients who underwent CPETs for varied reasons. We attempted to remove confounding factors such as age, gender, weight and height by calculating Peak VO2/kilogram of body weight and using percent predicted spirometry values.
We found that mid-expiratory flow FEF25-75% is highly predictive of low breathing reserve at peak exercise. FEF25-75% gives an estimate of average flow over the mid-portion of the forced maximal expiration and may be the most reliable marker of exercise related expiratory flow obstruction [25-28]. It has been suggested that low FEF25-75 represents significant obstruction in smaller conducting peripheral bronchioles which may be the predominant site of airflow limitation during exercise in common lung diseases like COPD, asthma, bronchiolitis or interstitial lung diseases with bronchiolar involvement [25-28].
Low-technology exercise tests including six minute walk testing and stair climbing can be done in outpatient settings to estimate a patient's actual peak VO2 without requiring the specialized personnel or equipment necessary for CPETs [29-31]. For example, the cut-off of 22 meters on stair climbing has been shown to have a positive predictive value of 86% for a VO2 peak of ≥ 15 ml/Kg/min; while the majority of patients unable to reach 12 meters had a VO2 peak below 15 ml/Kg/min [31]. The probability of a ventilatory limitation causing reduced exercise capacity can be easily calculated using mid-expiratory flow rates (FEF25-75%) in simple predictive tables (like Table 2 and Table 3). This may help clinicians utilize existing data to broaden or narrow the differential diagnosis of a patient's dyspnea and also can help guide the decision regarding the need for a CPET.
Table 2: Mid-expiratory flow FEF 25-75% predicted and absence of ventilatory limitation (Ventilatory reserve VR > 15%). View Table 2
Table 3: Use of FEF 25-75% to predict combined outcome (CO) of ventilatory limitation (VR ≤ 15%) & reduced exercise capacity; and low VR (≤ 15%) at peak exercise. View Table 3
Our study is the first to examine the association between baseline spirometry parameters and ventilatory limitations to exercise in all patients irrespective of the type of pulmonary disease and concomitant non-pulmonary co-morbidities. Mid-expiratory flow FEF 25-75% is closely associated with both pulmonary ventilatory limitation to exercise and the combined outcome of reduced exercise capacity and ventilatory limitation to exercise. Our observations showed that in those with FEV25-75% ≥ 40%, exercise limitation is much more commonly non-ventilatory in nature. In patients with mid-expiratory flow, FEV25-75% ≤ 20%, nearly 7 of 10 patients had ventilatory limitations to exercise. In patients with mid-expiratory flow, FEV25-75% ≤ 10%, nearly 7 of 10 patients had ventilatory limitation and low exercise capacity.
While ventilatory issues are commonly the cause of exercise limitation in patients with advanced lung disease, our results suggest that non-pulmonary co-morbidities continue to play an important role even in those with severe abnormalities in spirometry measurements.
Mid-expiratory flow below 20% predicted can be considered as cutoff to predict high probability of exercise related ventilatory limitation. Mid-expiratory flow below 10% predicted can be used as high probability of combination of low ventilatory reserve and low exercise capacity. Larger studies across multiple centers are required to verify our findings and test our predictive model.
We acknowledge the respiratory staff at St.Elizabeth Medical Center Pulmonary laboratory who assisted in the collection of this retrospective cardiopulmonary exercise test and spirometry data.
This was a retrospective review of cardiopulmonary exercise test and spirometry data. Patient identifying information was not recorded or reported anytime during our study. The Institutional Review Board (IRB) at St. Elizabeth Medical Center reviewed our study protocol and granted a consent waiver for our study.
We, the authors, do not have any financial interests (stocks, patents, employment, honoraria, or royalties) or nonfinancial relationships (political, personal, or professional) that may be interpreted as having influenced the writing of the manuscript.
No funding source was associated with our study.
Dr. Mandeep Hundal was involved in all steps of this retrospective review and manuscript preparation including literature review, data collection, analysis and manuscript writing. Dr. Christian Ghattas assisted with literature review, data collection and manuscript preparation. Lori Lyn Price was in-charge of statistics and data review. Dr. Peter Lacamera and Dr. John Unterborn were the guiding mentors involved in the project design, data review and manuscript preparation.