International Journal of Respiratory and Pulmonary Medicine
Functional Characteristics of COPD Patients Admitted for Acute Pulmonary Embolism
Rodríguez DA1* Orozco-Levi M1,2, Miranda F3, Mayoral A1, Clements JA1,4, Martínez-Llorens J1, Ventín C1, Bruguera J3, Gea J1 and Molina LL3
1Pulmonology Department, Hospital del Mar, Institut Hospital del Mar d'Investigacions Mèdiques, Universitat Pompeu Fabra, Spain
2Pulmonology Department, Fundación Cardiovascular de Colombia, Colombia, USA
3Cardiology Department, Hospital del Mar, Institut Hospital del Mar d'Investigacions Mèdiques, Universitat Pompeu Fabra, Spain
4Department of Family Medicine, University of Ottawa Bruyère Family Medical Centre Ottawa, Canada
*Corresponding author: Diego A. Rodríguez, Servei de Pneumologia, Hospital del Mar, Passeig Maritim 25 08003 Barcelona, Spain, Tel: 34-93-2483138, Fax: 34-93-2483425, E-mail: darodriguez@parcdesalutmar.cat
Int J Respir Pulm Med, IJRPM-1-003, (Volume 1, Issue 1), Research Article; ISSN: 2378-3516
Received: August 21, 2014 | Accepted: September 08, 2014 | Published: September 10, 2014
Citation: Rodríguez DA, Orozco-Levi M, Miranda F, Mayoral A, Clements JA, et al. (2014) Functional Characteristics of COPD Patients Admitted for Acute Pulmonary Embolism. Int J Respir Pulm Med 1:003. 10.23937/2378-3516/1410003
Copyright: © 2014 Rodríguez DA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction: Chronic Obstructive Pulmonary Disease (COPD) is a known risk factor for pulmonary embolism (PE); however, neither the clinical nor the pulmonary function characteristics are well described in COPD patients admitted for PE.
methods: We conducted a retrospective cohort study of 395 patients admitted for acute PE in a tertiary hospital setting. In COPD patients, clinical characteristics and pulmonary function were compared between the survivor and non-survivor groups during a 3-month follow-up period after PE.
Results: Thirty-three patients (8.3%) had previously documented diagnoses of COPD with moderate to severe airflow obstruction at least 6 months prior to the development of PE. The total number of deaths after three months of follow-up was 9 (27%) in COPD patients and 65 (17%) in patients without COPD (p = 0.03). PE was the leading cause of death in COPD. Only 15% of COPD patients had previous frequent exacerbations. The diffusion lung capacity for carbon monoxide (DLco, % predicted) was the only statistically significant variable that differed between the survivor and non-survivor groups of COPD patients (p = 0.002). The non-survivor group had decreased DLco values, with a DLco equal to or lesser than 60% being the best predictive value of mortality in these patients (AUC=0.88; p < 0.001).
Conclusion: COPD patients admitted for PE presented a higher risk of mortality than non-COPD patients. The COPD patient non-survivor group showed an important reduction of DLco prior to PE development. The degree of airflow obstruction; however, was similar between COPD survivors and non-survivors.
Keywords
COPD, Pulmonary Function, Pulmonary Embolism, Comorbidities
Introduction
Pulmonary embolism (PE) is a common cause of mortality, with an overall incidence rate of 69 cases per 100.000 inhabitants [1]. The clinical presentation and severity of PE are influenced by certain risk factors previously described in literature [2]. During the last decade, there has been increased evidence that chronic obstructive pulmonary disease (COPD) is a risk factor for Venous Thrombo Embolism (VTE) [3,4]. Moreover, COPD patients present more frequently with PE than with deep venous thrombosis (DVT) [6,7], which has been shown to contribute to the poorer prognoses in these patients [5-7]. Furthermore, similarities between the clinical manifestations of acute exacerbation of COPD (AECOPD) and PE have been shown to generate diagnostic challenges [8]. Currently, there is very little information available with regards to the functional respiratory characteristics of COPD patients admitted for PE and/or the clinical variables capable of predicting the clinical course of these patients [7]. The percentage of COPD patients admitted for PE does not exceed 10%, possibly due to the aforementioned diagnostic difficulties in this group [9,10]. According to recent studies though, despite the low percentage of COPD cases admitted for PE, these patients still have a higher risk of mortality [7]. Therefore, more information with regards to essential pulmonary functional characteristics and parameters of these patients would help to better diagnose and therefore rapidly identify therapeutic modalities for improved COPD patient survival.
Consequently, the objectives of this current study were: 1) to analyze the clinical and pulmonary function profile of COPD patients admitted for acute PE and; 2) to Identify predictors of mortality in this group of patients.
Methods
Study design and measurements
We conducted a retrospective cohort study of 395 patients admitted for acute PE between January 2007 and December 2011 in a tertiary hospital setting. Patients were classified using the Wells criteria as having low, moderate or high risk for PE, as described previously [11]. PEs were documented by either a positive helical computed tomography scan, a high-probability and/or an intermediate-probability ventilation–perfusion lung scan, a positive pulmonary angiography, or the visualization of a thrombus positioned in the right ventricle or right atrium on echocardiography [12]. Deep vein thrombosis (DVT) was diagnosed following acute symptoms of DVT and confirmed by compression ultrasound or contrast venography of the lower extremities. Furthermore, complementary information was collected, such as: demographic data, symptoms at presentation, the type of diagnostic method used, risk factors for DVT and information pertaining to treatment and complications. In particular, major bleeding complications were defined as either bleeding requiring transfusion of two or more units of blood or a fatal bleed. Moreover, immobilized patients were categorized under two different categories: 1) non-surgical patients who had been immobilized for =4 days; 2) immobilized surgical patients’, who had undergone a surgical procedure within last 2 months preceding PE development.
COPD was defined on the basis of smoking history and a post bronchodilator FEV1/FVC ratio less than 0.7 [13]. We included COPD patients who were both clinically stable and whose diagnoses were made at least 2 months prior to admission for PE in order to avoid over diagnosis.
Data analysis
Results are expressed as the mean ± standard deviation (SD) for normally distributed variables. Categorical data are reported as numbers and percentages. Comparisons between subsets of COPD patients (survivor and non-survivor groups) were performed using an unpaired T-test for continuous variables and a chi-square test for categorical variables. Furthermore, as comparisons retained only Diffusion Lung Capacity (DLco) (expressed as percentage of predicted) [14], a Receiver Operating Characteristic (ROC) analysis was performed with mortality as the “gold standard” reference in order to determine the best cut-off point for DLco during the 3 month follow up period.. Likewise, the area under the curve (AUC) was calculated for ROC curve non-parametrically [15,16]. The predictive values [17] were also calculated, both positive predictive value (PPV) and negative predictive value (NPV), in order to evaluate the best positive and negative results of the procedure. Afterwards, we explored the diagnostic capacity for prediction of DLco in the interval of 45% and 65% of the predicted value. Respective cut-off points were then selected that included the best sensitivity and specificity [18]. We also evaluated the means and the 95% confidence intervals (95% CI) for sensitivity, specificity, PPV and NPV. Calculations were done with SPSS/PC (version 18.0, SPSS Inc., Chicago, IL, USA). A p-value of < 0.05 was considered significant.
Results
From January 2007 and December 2011, a total of 395 consecutive adult patients with objectively confirmed acute PE were included in the study. Of these, 33 (8.3%) were diagnosed with COPD at least 2 months prior to admission.
The total number of deaths after 3 months of follow-up was 65 (17%) in non-COPD patients and 9 (27%) in COPD patients with (p = 0.03). PE was the major cause of death for COPD patients (5 out of 9 deaths, 56%), while AECOPD (n=2) and lung cancer (n=2) represented 44% of COPD patient mortality.
Table 1 demonstrates and compares the main characteristics of our COPD patient groups (survivor and non-survivor groups) throughout the follow-up period. . On average, the overall sample of patients showed a severe airflow obstruction with 30% of these patients being current smokers. Up to 15% of COPD patients had frequent exacerbations. The principal risk factors that correlated with the development of PE were obesity, immobilization and active cancer, with a total of 10 cancer patients assessed in the study (6 lung, 2 bladder, 1 stomach and 1 pancreas).
![]()
Table 1: Clinical and functional characteristics of COPD patients admitted for
acute pulmonary embolism (PE).
View Table 1
Tachycardia and dyspnea were the most frequent presenting clinical symptoms. Comparisons between the individual subset groups (survivor and non-survivor) showed the DLco (%predicted) to be the only statically significan differing variable among these two group subsets.
Figure 1 includes a ROC curve with the best DLco (% predicted) cut-off points for the evaluation y during the 3-month follow-up period, including respective AUC calculations (AUC= 0.88; p = 0.001).
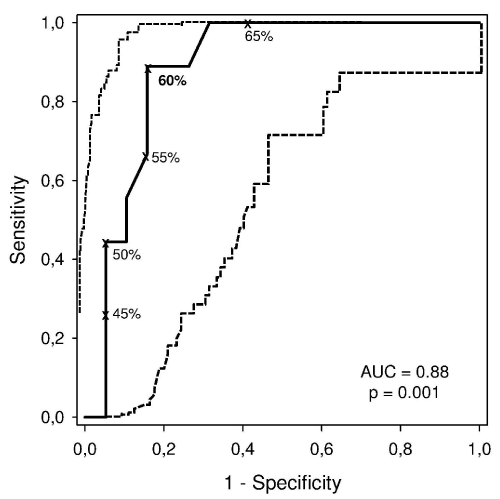 Figure 1: Receiver operating characteristic (ROC) curve (continuous line)
with 95% CIs (dashed line) for the various DLco (as % predicted) cutoff
points for death from any cause is shown. The best value (in bold) for
mortality prediction from any cause was 60%. AUC: area under the curve.
View Figure 1
Figure 1: Receiver operating characteristic (ROC) curve (continuous line)
with 95% CIs (dashed line) for the various DLco (as % predicted) cutoff
points for death from any cause is shown. The best value (in bold) for
mortality prediction from any cause was 60%. AUC: area under the curve.
View Figure 1
Table 2 demonstrates the values for sensitivity, PPV specificity and NPV for predicting mortality and indicates that a DLco equal to 60% is the threshold with the greatest capacity for predicting mortality (sensitivity: 0.88; PPV: 0.61; specificity: 0.80; NPV: 0.95).
![]() Table 2: Validity of the variables (means and 95% CI) for various DLco cut-points
for predicting mortality. The value in bold indicates the selected cut-point.
View Table 2
Table 2: Validity of the variables (means and 95% CI) for various DLco cut-points
for predicting mortality. The value in bold indicates the selected cut-point.
View Table 2
Regarding initial PE treatment, one patient received thrombolytic therapy, while one other patient received an inferior vena cava filter. Initially, all patients were treated with low molecular weight heparin (LMWH) for 5 days. Afterwards, COPD patients continued treatment with VKA throughout the next 3 months.
Discussion
To our knowledge this is the first study reporting clinical and respiratory functional characteristics of COPD patients admitted for PE. The main results demonstrate that while clinically stable, the pulmonary function parameters of these patients preceding PE development show: 1) severe airflow limitation and 2) a significant reduction of diffusion lung capacity for carbon monoxide in the non-survivor COPD patient who died during the 3-months follow-up compared to the COPD survivor group. Another important observation was that only 15% of COPD patients admitted for PE had frequent exacerbations.
Even though COPD patients with pe have been identified as a group of high risk mortality [7,8] , high risk bleeding and high risk vte recurrence [19] when compared to non-COPD patients developing pe, the clinical and pulmonary functional characteristics and parameters have still not been well explored in this population [7].
In our patients, low fev1, frequent exacerbations and/or increased comorbidities [13] did not represent high-risk factors. This study did identify; however, low dlco as being a a potential mortality risk factor. Low dlco has been generally described in patients with emphysema [13]. A decrease in size of the gas exchange area of the lungs and ventilation-perfusion mismatching leads to a reduction in dlco in this subtype of COPD patients [20,21]. Additionally, there are known associations between reduced dlco and clinical conditions such as acute pe [22,23]. This underlying mechanism could furthermore involve a reduced blood volume in the pulmonary capillaries [24]. Chronic pulmonary embolism, primary pulmonary hypertension (pph) and other pulmonary vascular diseases can also result in a decline in dlco [25]. For these reasons, an objective reduction of dlco prior to pe admission may explain the results of this current study.
We consider various factors of our study to provide relevant implications not only for future research but as well as clinical management and stratification of COPD patients with pe. Firstly, the initial clinical evaluations of COPD patients, including the calculations for pe risk stratification, are usually based on classical scales [11,26]. However, these scales do not take into consideration the severity of disease (i.e. Airflow limitation, dyspnea, etc.) Of COPD patients prior to pe development. This latter aspect was specifically researched throughout this study. Secondly, this study demonstrates that an adequate risk analysis could be beneficial for the improvement of individualized strategies on prevention, treatment and even follow-up following pe in this particular patient population .
The present study does though carry a series of limitations, among which is the size of the patient sample, its gender bias, as well as its retrospective nature. It should also be mentioned that lack of information concerning the degree of emphysema or presence of pulmonary hypertension could possibly have impacted the result interpretation of this study. . However, these limitations were also offset by two important strengths: 1) patients included in this study represented a homogenous group with a confirmed diagnosis COPD, which avoided possible over diagnosis; and 2) all of the pulmonary function studies were performed in the same laboratory, using a common systematic methodology.
In conclusion, the present findings showed that COPD patients admitted for pe have an elevated mortality when compared to non-COPD patients. Moreover, this study demonstrated for the first time, that COPD mortality from pe was associated with a manifested reduction in dlco prior to admission when compared to COPD survivors post-pe.
The present study constitutes a first attempt to increase our understanding of the complexity of pe pathogenesis in COPD patients. Future multicentric investigations though are warranted in order to confirm and expand on this study`s findings.
Acknowledgements
The authors would like to thank Angela Roig, Laura Gutierrez Martin, Nuria Bas Costas, Alba Ramirez-Sarmiento, Anna Herranz, Anna Rodó-Pin and Neus Bofil Soler for their collaboration throughout the study.
References
-
Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, et al. (1998) Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med 158: 585-593.
-
Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon WM, et al. (1999) Predictors of survival after deep vein thrombosis and pulmonary embolism: a population-based, cohort study. Arch Intern Med 159: 445-453.
-
Stein PD, Beemath A, Meyers FA, Olson RE (2007) Pulmonary embolism and deep venous thrombosis in hospitalized adults with chronic obstructive pulmonary disease. J Cardiovasc Med 8: 253-257.
-
Shetty R, Seddighzadeh A, Piazza G, Goldhaber SZ. Chronic (2008) obstructive pulmonary disease and deep vein thrombosis: a prevalent combination. J Thromb Thrombolysis 26: 35-40.
-
Fernandez C, Jimenez D, De MJ, Marti D, Diaz G, et al. (2009) [Chronic obstructive pulmonary disease in patients with acute symptomatic pulmonary embolism]. Arch Bronconeumol 45: 286-290.
-
Bertoletti L1, Quenet S, Mismetti P, Hernández L, Martín-Villasclaras JJ, et al. (2012) Clinical presentation and outcome of venous thromboembolism in COPD. Eur Respir J 39: 862-868.
-
Carson JL, Terrin ML, Duff A, Kelley MA (1196) Pulmonary embolism and mortality in patients with COPD. Chest 110: 1212-1219.
-
Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS (2006) Global burden of COPD: systematic review and meta-analysis. Eur Respir J 28: 523-532.
-
Tillie-Leblond I1, Marquette CH, Perez T, Scherpereel A, Zanetti C, et al. (2006) Pulmonary embolism in patients with unexplained exacerbation of chronic obstructive pulmonary disease: prevalence and risk factors. Ann Intern Med 144: 390-396.
-
Wells PS1, Anderson DR, Rodger M, Ginsberg JS, Kearon C (2000) Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost 83: 416-420.
-
Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED) (1999) The PIOPED Investigators. JAMA 263 :2753-2759.
-
Vestbo J1, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, et al. (2012) Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease, GOLD Executive Summary. Am J Respir Crit Care Med 187: 347-365.
-
Roca J, Rodriguez-Roisin R, Cobo E, Burgos F, Perez J, et.al, (1990) Single-breath carbon monoxide diffusing capacity prediction equations from a Mediterranean population. Am Rev Respir Dis 141: 1026-1032.
-
Hanley JA, McNeil BJ (1982) The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143: 29-36.
-
Hilgers RA (1991) Distribution-free confidence bounds for ROC curves. Methods Inf Med 30: 96-101.
-
Altman DG (1994) Bland JM. Diagnostic tests 2: Predictive values. BMJ 309: 102.
-
Altman DG, Bland JM (1994) Diagnostic tests. 1: Sensitivity and specificity. BMJ 308: 1552.
-
Curkendall SM1, Lanes S, de Luise C, Stang MR, Jones JK et al, (2006) Chronic obstructive pulmonary disease severity and cardiovascular outcomes. Eur J Epidemiol 21: 803-813.
-
Plummer AL (2008) The carbon monoxide diffusing capacity: clinical implications, coding, and documentation. Chest 134: 663-667.
-
Garcia-Aymerich J1, Gómez FP, Benet M, Farrero E, Basagaña X et al. (2011) Identification and prospective validation of clinically relevant chronic obstructive pulmonary disease (COPD) subtypes. Thorax 66: 430-437.
-
Liang BM, Lam DC, Feng YL (2012) Clinical applications of lung function tests: a revisit. Respirology 17: 611-619.
-
Prost JF, Desfonds P, Genevray B, Leroy M, Lacombe P (1984) [Evaluation of the severity of pulmonary embolism. Value of the measurement of stable carbon monoxide transfer capacity]. Presse Med 13: 1193-1197.
-
Hughes JM (2003) The single breath transfer factor (Tl,co) and the transfer coefficient (Kco): a window onto the pulmonary microcirculation. Clin Physiol Funct Imaging 23: 63-71.
-
Bernstein RJ, Ford RL, Clausen JL, Moser KM (1996) Membrane diffusion and capillary blood volume in chronic thromboembolic pulmonary hypertension. Chest 110: 1430-1436.
-
Moores L, Zamarro C, Gómez V, Aujesky D, García L, et al. (2012) Changes in pesi score predict mortality in intermediate-risk patients with acute pe. Eur Respir J 41: 354:359.
-
Rocha E, Jimenez D (2007) [Rebuttal of: "Can home prophylaxis for venous thromboembolism reduce mortality rates in patients with chronic obstructive pulmonary disease?"]. Arch Bronconeumol 43: 523-524.





