International Journal of Pathology and Clinical Research
The Optimization of Igm In-House ELISA for the Laboratory Diagnosis of Melioidosis in Malaysia
Azura Mohd Noor*, Norazah Ahmad and Wan Rozita Wan Mahiyuddin
Bacteriology Unit, Institute for Medical Research, Epidemiology and Biostatistic Unit, Institute for Medical Research, Kuala Lumpur, Malaysia
*Corresponding author:
Azura Mohd Noor, Bacteriology Unit, Infectious Diseases Research Center, Institute for Medical Research, Jalan Pahang, 50588 Kuala Lumpur, Malaysia, Tel: +60326162652, Fax: +60326919716, E-mail: azura.imr@gmail.com, amohdnoor1@sheffield.ac.uk
Int J Pathol Clin Res, IJPCR-1-007, (Volume 1, Issue 1), Research Article; ISSN: 2469-5807
Received: July 02, 2015 | Accepted: August 28, 2015 | Published: September 02, 2015
Citation: Noor AM, Ahmad N, Mahiyuddin WRW (2015) The Optimization of Igm In-House ELISA for the Laboratory Diagnosis of Melioidosis in Malaysia. Int J Pathol Clin Res 1:007. 10.23937/2469-5807/1510007
Copyright: © 2015 Noor AM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Melioidosis is a life-threatening infectious disease which can lead to high mortality and death within 48 hours if treatment is delayed. Detection of antibodies against Burkholderia pseudomallei may help in early diagnosis of melioidosis. This study was carried out to optimize an in-house ELISA for the detection of Immunoglobulin M (IgM) against B. pseudomallei in suspected acute melioidosis patients in Malaysia. Three different concentrations of crude B. pseudomallei cells antigen (0.200 mg/ml, 0.100 mg/ml and 0.05 mg/ml) and 2 different dilutions of conjugate (1:2000 and 1:4000) were used. The ELISA worked well with 0.05 mg/ml antigen when conjugated with 1:4000 peroxidase-conjugated goat anti-human-IgM. A total of 248 serum samples from 45 culture-confirmed melioidosis patients, 117 non-melioidosis patients (leptospirosis (n = 18), tuberculosis (n = 8), rheumathoid arthritis (n = 37), dengue (n = 12), hepatitis B (n = 23) and chikungunya (n = 19), and 86 healthy blood donors were used to construct receiver operating characteristics (ROC) curves. Based on the ROC analysis, this in-house IgM ELISA showed the highest sensitivity and specificity up to 82.2% and 94.87% respectively at the cut-off 0.605 serum absorbance. At this cut-off value, only 6 false positives were detected among 117 non-melioidosis patients, and the remaining 111 were detected as true negative. In conclusion, this in-house IgM ELISA can be applied for the laboratory diagnosis of acute melioidosis in Malaysian hospitals.
Keywords
IgM ELISA, Burkholderia pseudomallei, Melioidosis
Introduction
Melioidosis is an endemic disease in Malaysia and may cause death to patients if there is a delay in treatment. The disease is caused by soil saprophyte Burkholderia pseudomallei which is often isolated in paddy field soil and newly planted oil palm-areas, especially during a rainy-season [1]. The pathogen was reported having a long period of latency (26 years), in an infected host [2]. Positive serologic screening towards B. pseudomallei antibodies was detected in healthy human sera in the most areas of Malaysia and higher seroprevalence were observed among army recruits who served in several states of Peninsular Malaysia and Sabah [3].
Primary infection of melioidosis occurs through direct penetration of the pathogen into any cutaneous injury or skin abrasion of the host. The infection can also occur through inhalation [4] (website:http://www.cfsph.iastate.edu). People with diabetes are more prone to get the infection as it is a risk factor of the melioidosis especially among elderly [5]. Melioidosis may present as localized infection or septicemia with varying clinical symptoms. Septicemia, which is often linked with pneumonia, is the major cause of death in melioidosis patients. Fatal cases have been recorded as high as 68%-90% within 48 hours after the onset of symptoms [6-9]. A study conducted in Kedah, a north area of Malaysia, reported that 50% of their melioidosis patients (whom involving) with multi-organ failure and disseminated sepsis in the liver, spleen and soft tissue often succumbed to the disease [10].
A culture of B. peudomallei is the gold method for clinical laboratory diagnosis of melioidosis. However, a culture may not be feasible, especially if it requires obtaining the bacteria from the internal organs and tissues. Furthermore, the results from the culture may only be obtained only after 3-4 days. This may delay treatment and result in the death of patients [11,12]. Misidentification as B. cepacia or Pseudomonas stutzeri has been reported due to unfamiliarity of laboratory personnel in identifying B. pseudomallei [2]. An accurate diagnosis of melioidosis is crucial as it requires prolonged antimicrobial therapy up to 20 weeks to ensure recovery [4, 13,14]. Therefore, a simple, rapid, sensitive and specific test to diagnose melioidosis at an early stage of the disease would be beneficial, so that early treatment can be given to the patients.
ELISA is one of the serologic methods widely used to detect B. pseudomallei antibodies in human sera [15-17]. Detection of Immunoglobulin M (IgM) and IgG antibodies was found to be predominant in suspected melioidosis patients especially among septicaemic and localized infections [18]. Previously, the detection of IgG antibodies was often used for diagnosing melioidosis patients who were under antibiotics maintenance or eradication therapy with previous melioidosis history [19].
On the other hand, IgM ELISA was used to detect an active melioidosis infection. It was also used to distinguish between acute, subclinical and past infections of the disease in the endemic areas [6,20]. Moreover, the IgM ELISA using crude bacterial cells as an antigen was found to give a higher diagnostic accuracy with high sensitivity and specificity in detecting B. pseudomallei antibodies in human sera [13,21].
The aim of this study is to develop an in-house IgM ELISA using crude B. pseudomallei antigen against B. pseudomallei in order to detect recent infections of melioidosis among the Malaysian population.
Materials and Methods
Serum samples
A total of 248 serum samples were included in this study; culture-positive melioidosis (n = 45), leptospirosis (n = 18), rheumatoid arthritis (n = 37), tuberculosis (n = 8), dengue (n = 12), chikungunya (n = 19), hepatitis B (n = 23), and healthy blood donors (n = 85). For the samples of healthy donors, sera sample were drawn from a group of army personnel, government servants and normal residents from various parts of Malaysia. Sera sample of culture-positive melioidosis were taken from patients in various hospitals in Malaysia. The sera were confirmed positive to IgM against B. pseudomallei antibody at minimum titer > 1:160 by an in-house IgM-Indirect Immunofluorescent Antibody (IFA) test. Fourteen randomly selected sera of culture-positive melioidosis and healthy sera were used in a pilot study. The aim was to determine an optimal antigen concentration, working conjugate concentration and optimal substrate incubation period for the in-house IgM ELISA. All the serum samples were stored at -20°C until use. In the pilot study, the overall performance of the in-house IgM Elisa was tested against all serum samples.
Preparation of antigen
Three different isolates of B. pseudomallei B124/IMR, B125/IMR and B126/IMR isolated from blood of suspected melioidosis patients were used as the antigen for the in-house IgM ELISA. The isolates were confirmed as B. pseudomallei based on colonial morphology, standard biochemical tests, API 20NE and polymerase chain reaction (PCR) [22]. These isolates were obtained from different origin of Malaysia. They comprised of different fingerprint types by PFGE [5].
Several loops of single colonies of fresh cultured B. pseudomallei were suspended into 3 ml sterile distilled water then boiled at 100°C for 15 minutes. The boiled suspension was centrifuged at 3000 rpm for 15 minutes and the supernatant was removed. The killed bacterial cells were then washed out from debris cells by centrifuging it in phosphate buffer saline (PBS, pH7.2) at 3000 rpm for 15 minutes and repeated for five times. The washed cell pellets were kept in PBS buffer (pH 8.2) as antigen stock after its concentration was taken at 610 nm using spectrophotometer then stored at 4°C. For the working antigen, stock antigens were diluted with PBS buffer (pH 8.2) to prepare 0.05 mg/ml, 0.100 mg/ml and 0.200 mg/ml antigen. These three working antigen were prepared for isolates of B124/IMR, B125/IMR and B126/IMR. The amount of antigen in the buffer was determined using a spectrophotometer (SECOMAM) according to manufacturer's instructions. One milliliter of 0.05 mg/ml working antigen of each isolates, B124, B125 and B126 was mixed thoroughly to make 3 ml pooled antigen; then 75 μl of the mixture was coated into each well of a sterile 96 wells of immuno-plate. The same procedure was performed for the 0.100 mg/ml and 0.200 mg/ml working antigens. The wells were left to dry at 30°C then were sealed and stored at room temperature (32°C ± 2).
Preparation of testing sera
All of the 96 antigen-coated wells were blocked with 100 μl of blocking buffer (pH 7.2) (NaCl+NaHPO4+Tween 20) and 5 μl of freshly prepared skim milk and incubated at 37°C for 1 hour. The buffer-skim milk was removed, and the wells were washed 3 times with washing buffer (PBS pH 7.2 and 20% Tween 20). The plate was tapped on the absorbent paper to remove any excesses buffer in the wells. A total of 100 μl dilution buffer (0.05 % BSA, 20% Tween 20, pH 7.2) was added into each well except for the first row of wells. To prepare 1:80 sera dilution; 2.5 μl of tested sera was mixed with 197.5 μl of dilution buffer and transferred into wells in the first row of plate. One hundred microliters of the 1:80 diluted serum was transferred into a second row of the wells to make 1:160 dilutions, then serially diluted (two-fold) until 1: 81920. Each of the wells contained an equal volume of the respective diluted sera. For reagent control (blank), the wells were only added with 100 μl of dilution buffer. The plate was incubated at 37°C for 30 minutes then was washed 5 times with washing buffer. Excess buffer was removed by gently tapping the plate on the sorbent paper.
Preparation of conjugate and substrate
One hundred microliters of freshly prepared conjugate (peroxidase-conjugated goat anti-human IgM (KPL, USA)) of dilution 1:2000 or 1:4000 was added into the previous wells, and incubated at 37°C for 60 minutes. Each ELISA assay was performed in duplicate for 2 different conjugate dilutions. The wells were then washed 5 times with washing buffer and left to dry at room temperature for 5 minutes. A freshly prepared substrate containing 5-Aminosalicylic acid (5-AS) in 1% hydrogen peroxide (H2O2) was added into the wells, and the plate was immediately covered with aluminum foil. The plate was shaken gently and then incubated in a dark place at room temperature (32°C ± 2). Absorbance in each well was read in 30 minutes, 60 minutes, 90 minutes and 120 minutes at 490 nm. Normal control sera were run together in each set of the tests.
Diagnostic accuracy
Receiver operating characteristics (ROC) analysis is a widely-used method for comparing diagnostic accuracy of laboratory tests. It is constructed by plotting the sensitivity (true positive rate) on the y-axis against the 1-specificity (false positive rate) on the x-axis.
ROC is used to evaluate the performance of a diagnostic test in classification of subjects into two categories such as disease and non-disease [23,24]. If the area under the curve (AUC) is near to 1, it has higher chance of correctly classify the disease and non-disease groups. If the AUC is near to 0, it has a higher chance of incorrectly classify the groups.
A true positive melioidosis and true negative melioidosis groups were defined assuming that culture method is 100% sensitive and specific. The in-house IgM-ELISA absorbance of 45 positive melioidosis and 85 normal control on various sera dilutions were used to construct ROC. Based on the ROC, the best AUC was determined to select an optimal cutoff for positive-disease marker of melioidosis. The ROC, AUC, sensitivity and specificity of the in-house IgM ELISA were determined using SPSS Version 17.0. The statistical significance of difference of mean was assessed using One Way ANOVA test for comparison of three groups which were positive melioidosis, control and cut off point at each dilution. Post hoc analysis was carried out for further comparisons between each group. The level of significant was designated as p < 0.05. Values of AUC for ROC closer to 100% indicate better discrimination power between melioidosis and control.
Results
ELISA optimization
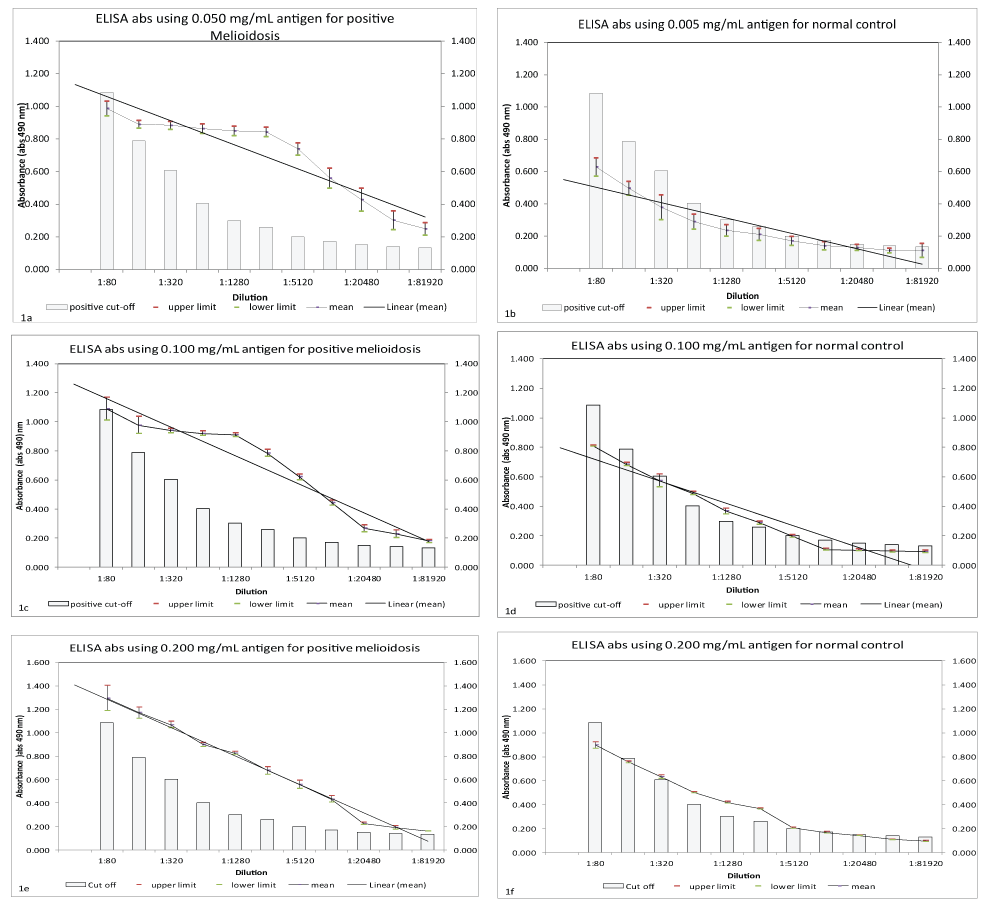
The optimized in-house IgM ELISA showed higher absorbance in culture-positive melioidosis at all the concentrations of antigen compared to normal control sera as shown in figure 1a, figure 1b, figure 1c, figure 1d, figure 1e and figure 1f. It was noted that ELISA absorbance for both types of sera showed decreasing trend over the sera dilutions. However, at antigen concentration of 0.05 mg/mL, larger percentage absorbance differences were detected between positive melioidosis and normal control as referred to (Figure 1a, Figure 1b, Figure 1c, Figure 1d, Figure 1e and Figure 1f).
.
Figure 1 (a-f): Antigen optimization using three different antigen concentrations (0.050 mg/mL, 0.100 mg/mL and 0.200 mg/mL); affected ELISA absorbance of positive melioidosis and normal control sera. (positive melioidosis n=7, normal control n=7).
View Figure 1 (a-f)
Further optimization of conjugate dilutions was based on 0.05 mg/mL antigen and the result showed an optimal conjugate dilution was 1:4000. At this conjugate dilution, larger absorbance differences between positive melioidosis and normal control were detected as shown in Table 1. At the dilution 1:320 until 1:5120 of sera, positive melioidosis was 50% different to normal control sera. Absorbance of the in-house ELISA, which was taken at 4 different incubation period after adding substrate 5-Aminosalicylic acid (5-AS) showed differences in readings for positive melioidosis case and normal contol. It was found that at 60 minutes after adding the substrate absorbance between positive melioidosis and normal control was greatly difference along all the sera dilutions as shown in Table 1. As for the negative control (reagent without any serum), the color did not changed during the duration of the incubation.
![]()
Table 1: The effect of conjugate dilution on ELISA absorbance for positive melioidosis and normal control sera at 60 minutes after adding substrate and the antigen concentration was 0.050 mg/mL
View Table 1
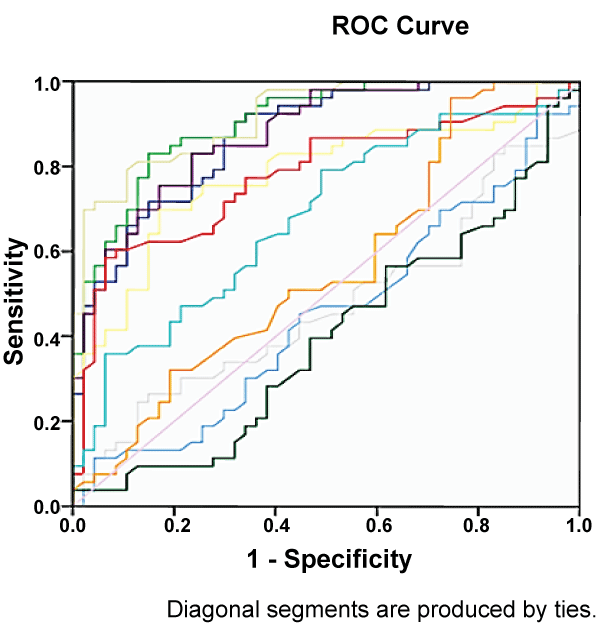
Figure 2 shows the ROC of all tested sera dilutions based on absorbance reading from 85 normal control and positive melioidosis sera. Based on the ROC analysis only 6 sera dilutions showed AUC at 0.85 and above as shown in Table 2. Each of it was further analysed to determine the working positive cutoff.
.
Figure 2: Receiver Operating Curve (ROC) plots for 11 different serum dilutions. The ROC was constructed based on 130 serum samples from melioidosis cases (n=45), normal control (n=85).
View Figure 2
![]()
Table 2: Area Under the Curve (AUC) at various serum dilutions, generated from ROC analysis on 130 serum samples from positive melioidosis (n=45) and normal control (n=85). The highest AUC was observed in dilution of 1:320
View Table 2
Positive cutoff for the in-house IgM ELISA was determined by AUC from ROC analysis using 45 positive melioidosis and 85 normal control sera. Table 2 shows a dilution of 1:320 showed the best AUC (0.970) with a cut-off point 0.605 (95% CI: 0.947, 0.993) (Table 2 and Table 4). One Way ANOVA and post hoc analysis showed that at 0.605 cutoff point the in-house ELISA could differentiate positive melioidosis and normal control sera significantly (p < 0.001).
![]()
Table 3: One Way ANOVA and Post hoc analysis to determine the optimal concentration of antigen for the optimized in-house IgM ELISA
View Table 3
![]()
Table 4: Sensitivity and specificity of the in-house IgM ELISA on six different sera dilutions with respective cutoff values using various types of sera samples
View Table 4
Six different in-house IgM ELISA exhibited the lowest false positive (6/117) at dilution 1:320. Sera sample of rheumatoid arthritis, chikungunya and leptospirosis often gave false positive results to the in-house IgM ELISA, probably there were cross reaction between antibodies of the disease and B. pseudomallei antigen. A summary of the sensitivity and specificity of the in-house IgM ELISA at different sera dilutions with respective cut-off values is shown in Table 2.
Discussion
ELISA is a method for detecting disease markers, allergens and proteins, including antibodies [25]. In this study, a direct antibody detection technique was chosen to optimize an in-house ELISA for detecting specific Imunoglobulin M antibody against B. pseudomallei in human sera.
This in-house IgM ELISA test showed higher specificity and sensitivity with the antigen concentration of 0.05 mg/ml, serum samples diluted in 1:320, conjugate dilution of 1:4000. The sensitivity and specificity were shown to be 82.2% and 94.87% respectively. The optimal concentration of crude antigen of B. pseudomallei for this in-house ELISA is at 0.05 mg/ml. At this concentration, all antigens bound well to the B. pseudomallei antibody. At higher antigen concentrations (0.100 mg/ml and 0.200 mg/ml), the antibody and conjugate were nonspecifically bound to the antigen surface, and the unoccupied sites of the antigen were fixed by another molecule of contaminant or reagents [26]. The amount of antigen is also a significant factor of the sensitivity of the test. The antigen, fixed onto the solid phase of the wells, is presumed as an antigenic cushion to capture the specific antibody. The optimal amount of the antigen will give high affinity in the binding of a complex antigen-antibody which will significantly affect the absorbance.
Sensitivity and specificity of the ELISA can be also affected by the quality of sera and antibody concentration. The testing serum must contain high concentration of antibody which will react specifically to the coated antigen. At lower concentrations of antibody (higher dilutions of testing sera), the absorbance of the test decreases considerably due to the formation of lower amounts of antigen-antibody complex (ELISA Technical guide and Protocols, Thermo Scientific). In this study, the optimal serum dilution was shown to be at 1:320.
The use of 5-AS as a substrate in this in-house ELISA gave more stability in absorbance reading. The optimal time for the incubation period for the substrate is 30 minutes. As compared to other studies which used o-phenylenediamine (OPD) as a substrate, the test reaction need to be stopped by adding a small amount of sulfuric acid (H2SO4) after a set time of incubation [16,27,28]. Although OPD produces a rapid color change in ELISA, it is toxic and proper precaution need to be taken to prevent direct contact with the substrate. Therefore, 5-AS is more preferred in routine ELISA. This is because it does not significantly change the P/N values after a longer incubation period, making the time of reading less critical [29].
Based on the findings above, this study showed that several factors can affect the outcome of the ELISA test. By optimizing the parameters, higher sensitivity and specificity can be achieved. Moreover, more reliable and accurate results can be obtained. This optimized in-house ELISA can be used as a serodiagnostic test for the detection of B. pseudomallei immunoglobulin M (IgM) in suspected acute melioidosis patients in Malaysia.
Acknowledgment
We thank the Director-General of Health, Malaysia for permission to publish this article. We would also like to thank the Director, Institute for Medical Research, Malaysia for her continuous support in preparing this article.
References
-
Strauss JM, Groves MG, Mariappan M, Ellison DW (1969) Melioidosis in Malaysia. II. Distribution of Pseudomonas pseudomallei in soil and surface water. Am J Trop Med Hyg 18: 698-702.
-
Amorn Leelarasamee (1986) Epidemiology of Melioidosis. J Infect Dis Antimicrob Agents 3: 84-93.
-
Strauss JM, Alexander AD, Rapmund G, Gan E, Dorsey AE (1969) Melioidosis in Malaysia. 3. Antibodies to Pseudomonas pseudomallei in the human population. Am J Trop Med Hyg 18: 703-707.
-
Cheng AC, Currie BJ (2005) Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev 18: 383-416.
-
Azura MN, Norazah A, Kamel AGM, Zorin SA (2011) DNA Fingerprinting of Septicemic And Localized Burkholderia Pseudomallei Isolates From Malaysian Patients. Southeast Asian J Trop Med Public Health 42: 114-121.
-
Ashdown LR, Johnson RW, Koehler JM, Cooney CA (1989) Enzyme-linked immunosorbent assay for the diagnosis of clinical and subclinical melioidosis. J Infect Dis 160: 253-260.
-
White NJ, Dance DA, Chaowagul W, Wattanagoon Y, Wuthiekanun V, et al. (1989) Halving of mortality of severe melioidosis by ceftazidime. Lancet 2: 697-701.
-
Sanford JP (1995) Pseudomonas species: including melioidosis and glander. In: Mandell GL, bennet JE and Dolin R Principles and Practice of Infectious Diseases. (4th edn) Churchill livingstone 1250-1254.
-
How SH, Ng KH, Jamalludin AR, Shah A, Rathor Y (2005) Melioidosis in Pahang, Malaysia. Med J Malaysia 60: 606-613.
-
HasHassan MR, Pani SP, Peng NP, Voralu K, Vijayalakshmi N, et al. (2010) Incidence, risk factors and clinical epidemiology of melioidosis: a complex socio-ecological emerging infectious disease in the Alor Setar region of Kedah, Malaysia. BMC Infect Dis. 10: 302.
-
Dharakul T, Songsivilai S, Anuntagool N, Chaowagul W, Wongbunnate S, et al. (1997) Diagnostic value of an antibody enzyme-linked immunosorbent assay using affinity-purified antigen in an area endemic for melioidosis. Am J Trop Med Hyg 56: 418-423.
-
Sirisinha S, Anuntagool N, Dharakul T, Ekpo P, Wongratanacheewin S, et al. (2000) Recent developments in laboratory diagnosis of melioidosis. Acta Trop 74: 235-245.
-
Narisara Chantratita, Vanaporn Wuthiekanun, Aunchalee Thanwisai, Direk Limmathurotsakul, Allen C. Cheng, et al. (2007) Day and Sharon Peacock. Accuracy of Enzyme-Linked Immunosorbent Assay Using Crude and Purified Antigens for Serodiagnosis of Melioidosis. Clin Vaccine Immunol 14: 110-113.
-
Wuthiekanun V, Peacock SJ (2006) Management of melioidosis. Expert Rev Anti Infect Ther 4: 445-455.
-
Phung LV, Han Y, Oka S, Hotta H, Smith MD, et al. (1995) Enzyme-linked immunosorbent assay (ELISA) using a glycolipid antigen for the serodiagnosis of melioidosis. FEMS Immunol Med Microbiol. 12: 259-64.
-
Rasana Wongratanacheewin S, Surasakdi Wongratanacheewin, Narisara Anuntagool , Stitaya Sirisinha (2000) Comparison of the Polymerase Chain Reaction and Serologic Tests for Diagnosis of Septicemic Melioidosis. Am J Trop Med Hyg 63: 146-149.
-
Chen YS, Shiuan D, Chen SC, Chye SM, Chen YL (2003) Recombinant truncated flagellin of Burkholderia pseudomallei as a molecular probe for diagnosis of melioidosis. Clin Diagn Lab Immunol 10: 423-425.
-
Chenthamarakshan V, Vadivelu J, Puthucheary SD (2001) Detection of immunoglobulins M and G using culture filtrate antigen of Burkholderia pseudomallei. Diagn Microbiol Infect Dis 39: 1-7.
-
Vasu C, Vadivelu J, Puthucheary SD (2003) The humoral immune response in melioidosis patients during therapy. Infection 31: 24-30.
-
Kunakorn M, Boonma P, Khupulsup K, Petchclai B (1990) Enzyme-linked immunosorbent assay for immunoglobulin M specific antibody for the diagnosis of melioidosis. J Clin Microbiol 28: 1249-1253.
-
Limmathurotsakul D, Chantratita N, Teerawattanasook N, Piriyagitpaiboon K, Thanwisai A, et al. (2011) Enzyme-linked immunosorbent assay for the diagnosis of melioidosis: better than we thought. Clin Infect Dis. 52: 1024-1028
-
Wuthiekanun V, Smith MD, Dance DA, Walsh AL, Pitt TL, et al. (1996) Biochemical characteristics of clinical and environmental isolates of Burkholderia pseudomallei. J Med Microbiol 45: 408-412.
-
Beck JR, Shultz EK (1986) The use of relative operating characteristic (ROC) curves in test performance evaluation. Arch Pathol Lab Med 110: 13-20.
-
Biao Zhang (2006) A semiparametric hypothesis testing procedure for the ROC curve area under a density ratio model. Computational Statistics and Data Analysis 50: 1855-1876.
-
Engvall E, Perlmann P (1971) Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry 8: 871-874.
-
Daniela Botus, Tatiana Oncescu (2006) Optimizing Immunoenzymatic Reactions (ELISA) for The Derection of Antibody Against NDV Virus. University of Bucharest, Faculty of Chemistry II: 33-41.
-
Zeinab A Demerdash, Tarek M Diab, Ibrahim R Aly, Salwa H Mohamed, Faten S Mahmoud, et al. (2011) Diagnostic efficacy of monoclonal antibody based sandwich enzyme linked immunosorbent assay (ELISA)for detection of Fasciola gigantica excretory/secretory antigens in both serum and stool. BMC Parasites and Vectors 4: 176.
-
Suat Moi Puah, SD Puthucheary, Kek Heng Chua (2013) Potential Immunogenic Polypeptides of Burkholderia pseudomallei Identified by Shotgun Expression Library and Evaluation of Their Efficacy for Serodiagnosis of Melioidosis. Int J Med Sci 10: 539-547.
-
Ellens DJ, Gielkens AL (1980) A simple method for the purification of 5-aminosalicylic acid. Application of the product as substrate in enzyme-linked immunosorbent assay (ELISA). J Immunol Methods 37: 325-332.





