The 21st century has been the period for immunotherapy for cancer, including the use of vaccines, developed for the control of infectious agents, and now showing its usefulness also for internal agents such as cells tumors that are cloned and have accelerated mitosis states.
At Cancer Research Laboratory of Manuela Beltran University (UMB), research was carried out with the design and development of the CIMT-54 biological vaccine: A combination of oncotropic virus + amino acids as adjuvants, which act directly on the tumor cell, preventing the reproduction of metastases to other organs and preventing the attack on healthy cells of the organism. The results at preclinical level suggested that the CIMT-54 vaccine is not cytotoxic; and on the other hand, in the studies carried out in Wistar rats, it was observed that the vaccine is capable of "blocking" the tumor cells, which makes it effective to stop the metastasis, respecting the healthy tissues and organs.
A clinical study was carried out with 59 individuals, of which 21 were patients with different stages of cancer, the majority previously treated with conventional therapy. A protocol of 9 doses of the CIMT-54 vaccine was initiated, without any other type of coadjuvant treatment (by decision of the patients), in order to evaluate the safety and efficacy in the different types of cancer, managing to demonstrate the clinical and paraclinical stability of the patients, with a low rate of progression, and also improving the quality of life of the patient.
Immunotherapy, Cancer, Vaccines, Oncolytic virus
In general terms, two major mechanisms of immunity to tumors can be defined: Humoral and cellular. An important aspect of both is the ability of antigen-presenting cells to process and present the tumor peptides that form the basis of the immunological recognition of tumor cells. The tumor antigens are phagocytized and partially digested by the antigen-presenting cells; they are presented as peptides bound to MHC type II receptors on the surface of the antigen-presenting cell. Antigen-presenting cells include macrophages, epidermal Langerhans cells, other dendritic cells, and B-lymphocytes. The MHC type I surface receptors that form the basis of HLA tissue typing are present in all nucleated cells of the body, including the tumor cells [1-4]. These receptors present, in a quasi-random manner, examples of intracellular peptides. The MHC type I receptors of the tumor cells also present tumor-specific peptide antigens, allowing the immune system to adequately sensitize to react against the tumor [5].
For decades, the use of conventional therapies such as surgery, chemotherapy, and radiotherapy have governed the management of cancer patients [6-9]. However, there are multiple cases where a total benefit of the patients is not achieved, with new tumor relapses and/or adverse effects attributable to said management [10]. Alternative treatments have been considered, which allow the effective elimination of tumor cells, minimizing adverse effects, as well as tumor relapse, within which immunotherapy has emerged, as an attractive strategy. Immunotherapy includes techniques that enhance the natural immune response to tumors, through vaccines or biological response modifiers, mainly immunomodulatory cytokines [11-14].
Cancer therapeutic vaccines are based on the manipulation of the immune system in order to complement traditional oncological treatments such as chemotherapy, radiotherapy and radical surgery or they can act on their own causing the specific immune response on tumor cells, developing the ability to fight against the advancement of cancer cells [15,16]. At present both preventive and therapeutic vaccines have been developed [8], and are in the stage of clinical experimentation, different types of vaccines from interferons (IFN alpha, beta and gamma), interleukins (IL-2), colony stimulants factors (CEF) and monoclonal antibodies (MAb) in various types of tumors [6,17-25] .
Vaccines are today a new therapeutic alternative to counteract not only cancer but most of the chronic infectious diseases that affect the world population [26-28]. Currently, there are more than 300 vaccine studies for different types of cancer. Experimentally, in the 1960s, Wheelock and Dingle [29] (NEJM, 1964), considering acute leukemia as an entity of viral origin, carried out a protocol for the administration of viruses such as Sendai, Newcastle and strains of Influenzae A and B with in order to achieve viral interference, denoting the decrease in production of myeloblasts, as well as hepatosplenic size reduction, but with very transient effect and non-sustained remission. A series of vaccines are being developed, some still in preclinical experimentation, while others are already being tested in clinical studies (Phases I, II, III and IV) [28], as in Argentina, where a vaccine called Vaccimel has been developed, for stages III and IV of melanoma with promising results, it is already being applied to a group of more than 100 patients in advanced stages [30].
There is also another type of immunotherapy, called passive [31,32], which consists of the use of the so-called Monoclonal antibodies, designed to bind specific targets called checkpoints, such as CTLA4, PD1 and PDL1, which are specific proteins and whose antibodies are anti CTLA4, anti PD1 and anti PDL1, which will cause these antibodies to bind to said proteins to block them through the T lymphocytes and the CMH of the dendritic cell, which is the antigen presenting cell-CPA [33-36]. However, this type of therapy has shown the presence of side effects, especially at the rheumatological and immunological level, but also in other systems, such as digestive, neurological, cardiopulmonary [37,38]. Similarly, cytokines (Il-2, Interferon) were the first drugs used as a cancer treatment, but in turn, due to their high toxicity, they are not the first choice [6,39]. Therefore, there is a need for the use of immunotherapy mediated by oncotic viruses, where safety has been demonstrated in induction of a directed immune response, low rate of adverse effects as well as a low risk of autoimmunity associated with the use of the vaccine, and a strong oncoreactivity for stop tumor progression, and in some cases complete remission [40-45]. Several types of viruses have been evaluated, with variable results in their outcomes; particularly in the UMB, a study of several types was initiated until obtaining the best response with an oncotropic virus of canine origin, together with adjuvant amino acids, the immune complex CIMT-54 (previous in vitro tests) was achieved 1778/8.
We recruited 59 individuals for the total study: 21 patients with different types of cancer and in different stages (I-IV) received the immune complex, in order to evaluate the Safety and Efficacy of the CIMT-54 vaccine, comparing its behavior with 24 patients with cancer who did not receive the immune complex (and continued their usual conventional management), as well as two control groups: 4 healthy controls immunized with the immune complex, and 10 healthy controls not immunized, between 2007 and 2008.
After acceptance by the Ethics Committee of Manuela Beltran University (UMB), held on February 22, 2007 (document 22022007), approving the participation of patients in compassionate experimental study after checking the results obtained in preclinical studies, patients were recruited to through television information on regional channels in the country, such as City TV, RCN and radio media such as W radio. It was explained to the media what the vaccine consisted of and the preclinical results that had been obtained.
A 2-hour conference was held in the UMB auditorium on how they could participate in the study, they have explained what the vaccine consisted of and the inclusion and exclusion criteria for the participants were evaluated (see Table 1).
The recruited patients signed informed consent to enter the study voluntarily. Once all the requirements were fulfilled and they were accepted in the study, a clinical history of admission was made with the laboratory examinations prior to the study. They have initially given three doses of 0.3 mL of a vaccine, subcutaneously weekly and then monthly reinforcements for 12 months.
Each patient was evaluated individually at 3 months from the start of treatment and then at 6, 9 and 12 months of the study with paraclinical studies (complete blood count, tumor antigens, liver tests [Transaminase, alkaline phosphatase, bilirubin], partial urine, as well as measuring Interleukins [IL-2, IL-6, IL-8, IL-10 and IL-15, as well as TNF-α] with BioLegend reagent kit [ELISA kit with human interleukins, San Diego, CA 92121]) for the analysis at admission with active tumor status (and also baseline measurement in immunized and non-immunized controls), and subsequent follow-up at 12 months. Additionally, studies of diagnostic images (MRI, CT, Rx, ultrasound) were ordered according to the type of cancer.
Safety was evaluated, defined as absence of side effects adverse to the use of the CIMT-54 vaccine at a dose of 0.3 ml determined in the in vitro study as the adequate dose for the patients in question, as well as the effectiveness of the product, defined as achieving a condition of stability (no changes) or improvement of the oncological process (partial remission or complete remission) according to WHO and RECIST criteria for response in solid and hematological tumors [46,47] (see Table 2). Follow-up of tumor markers in response was also performed [48,49], and interleukin monitoring throughout the entire process to determine the antitumor effect associated with the vaccine.
Of the 57 total cases, 68.4% were women and 31.6% men, average age 45.9 years (+/- 16), with breast cancer as predominant tumor (41.9%), in large percentage in advanced tumor stages (stage IV 28%, stage III 37%, see Table 2). Patients evaluated during and after treatment with the vaccine showed that it was safe, given the absence of side effects attributable to the use of the immune complex, and was effective in clinical and paraclinical stability (see Figure 1 and Figure 2), regardless of the tumor type and of the presented stadium. There was even a great response in terms of partial remission (improvement) and no changes (stability) in patients in stages III and IV (see Figures 3 and Figure 4), which is an interesting finding, given that some of the cases had already been declared "patients with no possibility of treatment in a conventional manner", due to the advanced stage of the disease. Additionally, the behavior of the Interleukins, at 12-month control, in cancer patients who received the immune complex, demonstrated a decrease in IL-2 expression (by 58% compared to sick patients not immunized), IL-6 (in 82% compared to sick patients not immunized), IL-8 (in 76% compared to sick patients not immunized), and TNF-α (in 75% compared to sick patients not immunized), being the cytokines keys in tumor growth, tumor progression, antitumor immune resistance as a mechanism of evasion, maintaining stable levels of IL-10 and IL-15 (promoters of antitumor cytotoxicity, as well as blocking tumor growth). See Figure 5.
Table 1: Inclusion and exclusion criteria. View Table 1
Table 2: Characterization of the population. View Table 2
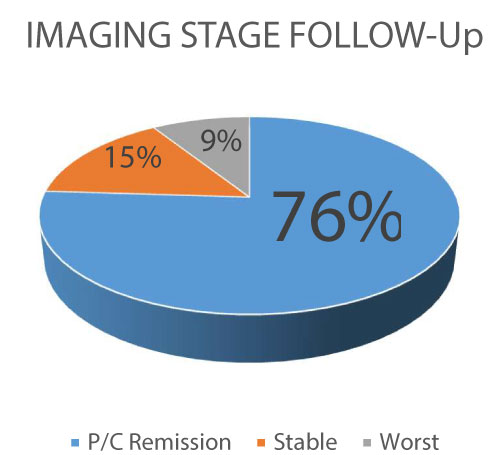 Figure 1: Imaging Follow-up (P/C: partial/complete).
A several group of patients keep on stability after the CIMT-54 use, with only a 9% of progression of the disease.
View Figure 1
Figure 1: Imaging Follow-up (P/C: partial/complete).
A several group of patients keep on stability after the CIMT-54 use, with only a 9% of progression of the disease.
View Figure 1
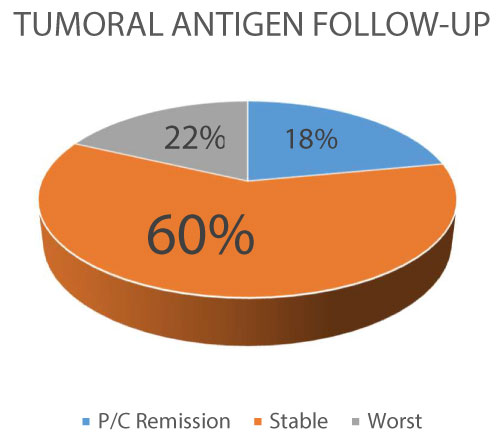 Figure 2: Tumoral antigen Follow-up (P/C: partial/complete).
Figure 2: Tumoral antigen Follow-up (P/C: partial/complete).
A 60% of the patients reach stability of the tumoral antigens as a marker of control of the inflammatory activity of the cancer, with a 22% with progression (worst).
View Figure 2
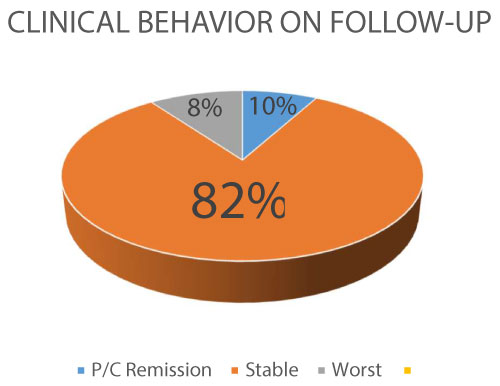 Figure 3: Clinical Follow-up (P/C: partial/complete).
Figure 3: Clinical Follow-up (P/C: partial/complete).
In general, the clinical behavior of the patients match criteria for stability in 82% of the cases, with a 8% of progression.
View Figure 3
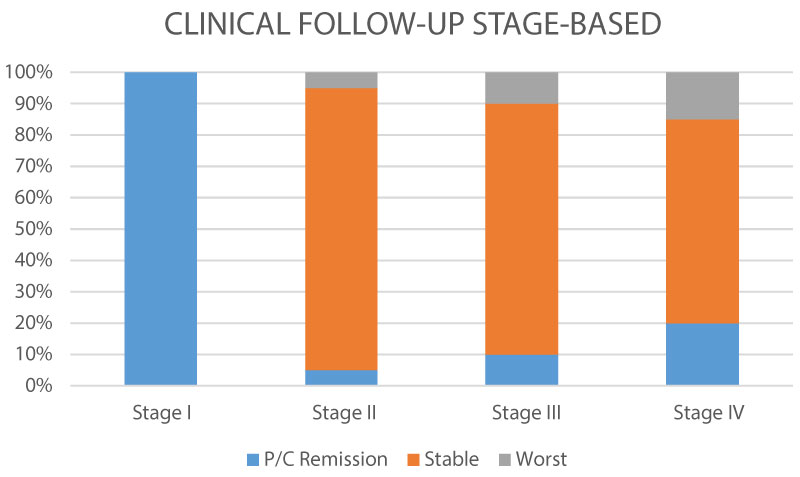 Figure 4: Clinical behavior Tumor stage-based (P/C: partial/complete).
Figure 4: Clinical behavior Tumor stage-based (P/C: partial/complete).
Comparing tumoral stages, we observed that the clinical stability was similar, with a low rate of progression, more evident in advanced stage (IV), and with total remission en early stage (I).
View Figure 4
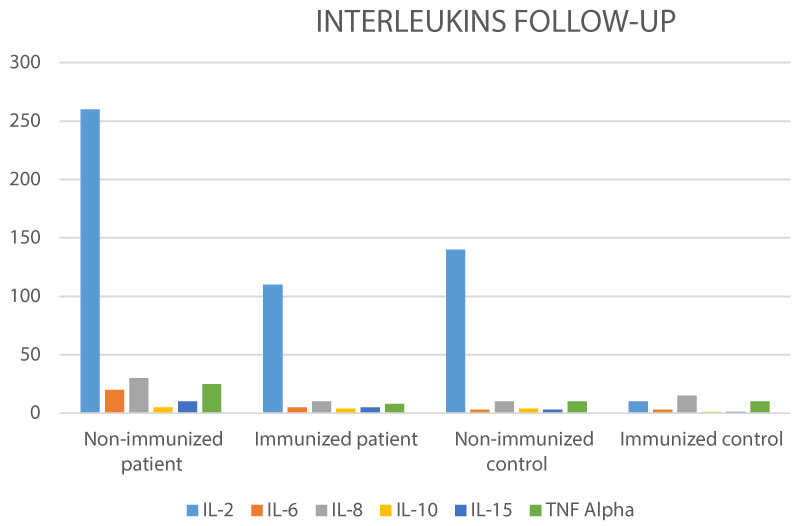 Figure 5: Interleukin's (pg/dL) on patient's vs. controls Follow-up.
Figure 5: Interleukin's (pg/dL) on patient's vs. controls Follow-up.
A comparison between IL-s between patients immunized and non-immunized, demonstrate that CIMT-54 reduce the expression of IL-2, IL-6, IL-8, as a markers of inflammatory tumoral activity, with stability of IL-10, IL-15 as promoters of antitumor cytotoxicity.
View Figure 5
Previous studies performed in vitro tests with tumor cells show morphological evidence of cell adhesion and interruption of mitosis, stimulation of adhesion molecules (cadherins, adhesin, connexins) when the vaccine was applied in 3 doses, not allowing these cells they continued dividing by mitosis, thus avoiding the tumor progression towards metastasis.
84% of the patients in our study had received some type of conventional treatment (Chemotherapy, Radiotherapy and/or surgery), while 16% on admission had not received any treatment, regardless of the stage in which they were.
The established dose/response of the vaccine was 0.3 mL. This was applied to the group of patients, with no noticeable side effects or anaphylactic reactions. The CIMT-54 vaccine was applied in three initial doses, with an interval of 1 week between each and with monthly reinforcements, in a period of 12 months, observing a promising response that allowed to project the efficacy of the CIMT-54 vaccine and justify the clinical trial that we call a case study, because, with the results in preclinical phase that was also very promising, it was concluded that the CIMT-54 vaccine was not cytotoxic in the in vitro studies, nor toxic in the experimental animals at the dose 0.3 mL, after sufficient tests were performed that allowed the adjustment of the dose/response.
The administration of 9 doses of the CIMT-54 vaccine at the established dose, without other coadjuvant treatment, allows us to infer that the treatment with the CIMT-54 vaccine was successful, both in prolonging and improving the quality of life of the patient, without receiving no complementary treatment, as well as maintaining clinical and paraclinical stability indexes, which would make it possible for the CIMT-54 vaccine to be an effective and economical alternative in the treatment of cancer, especially in those cases of early detection, finding an scientific option for patients in terminal stages [50,51]. We believe that therapy with the CIMT-54 vaccine is promising to prevent and treat cancer. The limitations in our study are focused on the few immunological tests that were carried out, given technical and logistical difficulties with higher technology equipment, which would give a much broader richness to the beneficial effects of the vaccine, as well as allow us under a more detailed analysis, the possibility of evaluating which type of tumors can have a greater response based on the oncotrophic mechanism of the virus, and by which routes.
There are several questions:
1. What type of immunotherapy would be the most appropriate for patients with cancer?
2. Having shown in this case that vaccines have fewer side effects for cancer patients, could they be more effective than monoclonal antibodies?
3. The problem with vaccines for cancers takes much longer to produce a response and be effective than chemotherapy itself. Could this be a limitation for its use?
4. Could the side effects of monoclonal antibodies be improved to make them more effective?
5. Would vaccines reduce treatment costs and allow a greater field of action, unlike monoclonal antibodies, taking into account the direct costs, accessibility, and barriers of the health system?
The authors declare that they have no competing interests.