The purpose of this review article is to examine the etiology, incidence and classification of white spot lesions in association with their prevention and treatment methods. White spot lesions are opacities that occur by demineralization of enamel under the surface and cause esthetic problems. Orthodontic treatment increases the risk of white spot lesion occurrence by creating areas difficult to clean and prone to plaque accumulation around orthodontic attachments. Therefore, application of suitable methods for prevention of these lesions during orthodontic treatment and performing appropriate therapies after orthodontic treatment is important for obtaining favorable results and patient satisfaction. Oral hygiene motivation, usage of topical fluoride agents, casein phosphopeptide-amorf calcium phosphate agents, antimicrobial agents, tooth bleaching, microabrasion and resin infiltration are current techniques that clinicians can use for prevention and treatment of white enamel lesions.
White spot lesion, Enamel demineralization, Plaque accumulation, Oral hygiene, Fluoride, CPP-ACP, Resin infiltration, Orthodontic treatment
Enamel decalcification occurs when bacterial flora remains on enamel surface for a long time [1]. Organic acids produced by bacteria enter interprismatic spaces in tooth enamel, resulting in white lesions due to dissolution of apatite crystals, calcium and phosphate ions, and demineralization [2].
Generally, enamel discoloration can be seen in the form of dental fluorosis, opacity and white spot lesions. Fluorosis is a white to yellowish colored lesion that is not completely distinguishable, fused with normal enamel and symmetrically distributed. On the other hand, opacities that are not fluoride-related have more specific shapes, differ from tooth enamel and are often found in the middle of teeth. In patients receiving orthodontic treatment, white spot lesions often occur beneath broken bands, around bracket bases, and in regions where brushing is difficult [3]. The purpose of this review article is to examine the etiology, incidence and classification of white spot lesions in association with their prevention and treatment methods.
The search strategy was organized across 4 electronic databases (Pubmed, Web of Science, Medline, Scopus) using search terms such as "orthodontic treatment", "white spot", "lesion", "white discoloration", "CPP-ACP" and "Fluoride". Abstracts for all relevant terms were retrieved and evaluated independently by 2 reviewers (S.S.T. and B.K.). Exclusion criteria were developed for study selection. Studies published before 1975 and not available in full text in English were excluded. Data selection was completed by one reviewer (S.S.T.) and checked for accuracy and consistency by a second reviewer (B.K.). Mendeley reference management software was used for organization of citations.
Adhesives around orthodontic attachments constitute areas that are suitable for increase in bacterial counts [4]. It was reported that following application of orthodontic appliances, a rapid change in bacterial composition of plaque was observed, and the amount of acidogenic bacteria such as Streptococcus mutans and Lactobacillus significantly increased. These bacteria reduce pH level of plaque in presence of fermentable carbohydrates. Decalcification occurs when the pH level of oral environment falls below the threshold level of remineralization [3].
Irregular composite residues around brackets increase plaque accumulation, but fluoride-containing or anti-microbial composites decrease the acidity level of oral environment and prevent demineralization. In general, white lesions due to orthodontic treatment occur on buccal surface of teeth and in areas that are difficult to clean (Figure 1). These are the areas between brackets and gingiva, especially in upper lateral and canine teeth and lower canine and first premolar teeth [1].
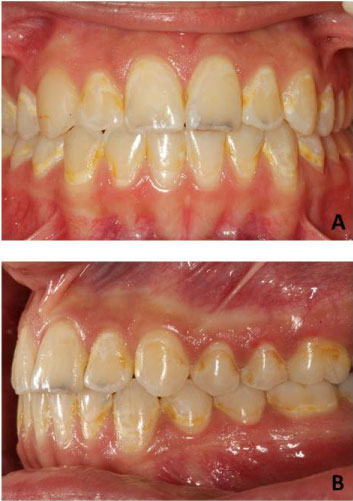 Figure 1: A patient presenting mild to severe white spot lesions in number of teeth after orthodontic treatment. A) Intraoral frontal view; B) Intraoral lateral view.
View Figure 1
Figure 1: A patient presenting mild to severe white spot lesions in number of teeth after orthodontic treatment. A) Intraoral frontal view; B) Intraoral lateral view.
View Figure 1
Gorelick, et al. [1] reported the factors affecting formation of white spot lesions as; surface characteristics of teeth, saliva access and distance between brackets and gingiva. No association between white lesion formation and lingual retainers was observed. This was explained with the protective role of saliva flow amount and buffering capacity against acid attacks.
Despite there are differences between the studies, the most affected teeth examined are upper lateral incisors, upper canines, lower canines and lower first molars, respectively [1].
White-spot lesions were first classified in orthodontics according to their visual size [5]. This classification made according to the width of opacity formed on enamel surface was as follows:
Class 0: None or less than 1 mm opacity
Class 1: Opacity covers 1/3 of tooth surface
Class 2: Opacity covers 1/3 to 2/3 of tooth surface
Class 3: Opacity covers wider than 2/3 of tooth surface
Another classification made by Gorelick, et al. [1], which considers both size and intensity of lesions is as follows:
Class 1: No white spot lesion formation
Class 2: Mild white spot lesion present
Class 3: Severe white spot lesion present
Class 4: Cavitation is present in addition to white spot lesion
Accurate and rapid evaluation of white spot lesions during orthodontic treatment is very important for performing protective, preventive and corrective therapies [6]. Assessment of white spot lesions requires two steps. The first step involves evaluation of decalcification and the second step involves evaluation of severity of a lesion. Severity is assessed according to the brightness and size of a discoloration macroscopically, whereas according to the amount of mineral loss and lesion depth microscopically [6].
The structure of hydroxyapatite crystals is under the influence of a natural cycle between demineralization and remineralization. This cycle works in the favor of either demineralization or remineralization, depending on environmental factors [7]. Studies using polarized light microscopy indicate that the size of white lesions may decreases over time [8]. There are studies showing that white lesions resulting from orthodontic treatment decrease rapidly after removal of orthodontic appliances within 12 months and may decrease up to 50% within 24 months [8]. The degree of remineralization varies between individuals and different regions within the mouth. Sometimes the size of a demineralization area may be such that it cannot be improved even if an effective remineralization agent is used. Therefore, methods to prevent white lesion formation is important [8].
During orthodontic treatment, the best method is to prevent white spot lesions before they develop. There are two fundamental methods to accomplish this. The first one is to avoid breaking of ongoing demineralization or to strengthen remineralization process. The second one is to prevent demineralization on tooth surface [9].
Methods for increasing remineralization and decreasing demineralization in patients undergoing orthodontic treatment are; oral hygiene motivation, regular professional oral hygiene appointments, usage of topical agents and orthodontic adhesives involving fluoride [9].
Saliva is also very important information or prevention of tooth decay. Degradation of minerals in enamel after acid attacks, demineralization degree, the onset or duration of remineralization are related to the pH level of saliva [10].
The pH level around braces can easily decrease in patients receiving orthodontic treatment. If patient has good oral hygiene, pH level will not exceed the critical level in early stages of acid attacks. However, if patient's oral hygiene is poor, permanent mineral loss can be observed around braces, as these areas remain below critical pH level for a long time.
Reducing the amount of plaque on tooth surface is an effective method for preventing caries formation [11]. Removal of dental plaque is possible by mechanical and chemical methods. Tooth brushing is a commonly used method for mechanically controlling plaque. Mouthwashes involving different ingredients that provide chemical plaque control are also effective agents that reduce bacterial count by 99.9% without damaging the surrounding oral tissues. Mouthwashes containing chlorhexidine are the most effective [12].
Fluoride application is a commonly used method to reduce enamel tendency to demineralization. The concentration of fluoride in saliva and plaque is effective in prevention of demineralization and formation of remineralization [13]. The organic acids formed by cariogenic bacteria cause decrease in pH level of plaque, which results in fluoride diffusion into enamel from plaque and saliva in response. Displacement of hydroxyl ions of enamel structure with fluoride causes existence of fluorapatite crystals. This new crystal form is more resistant to acids [14]. Fluoride also affects the activities of cariogenic bacteria and prevents formation of caries. Laboratory studies have shown that low concentrations of fluoride cause Streptococcus mutans to produce less amount of acid [15].
The concentration of fluoride in enamel surface decreases dramatically with depth. Fluoride has low solubility and tends to accumulate on enamel surface. It cannot go deeper in enamel layers after porous cavities are filled with it. In a study, fluoride was applied for 3 months to a white lesion of 100 μm depth which was created in experimental conditions. Following 3 months of application, fluoride ions could only reach a depth of 50 μm [16]. Therefore, a low dose of fluoride is recommended for the fluoride ions to reach deeper layers of lesions easily. Lee Linton [17] claims that a mouthwash containing 50 ppm fluoride is more effective in remineralization than one that contains 250 ppm fluoride. On the other hand, according to the results of a clinical study published by Willmot, et al. [18], there is no additional remineralization effect of mouthwashes and toothpastes with low dose fluoride compared with the fluoride-free ones. Additionally, it is reported that the ability of fluoride ion to remineralize is less than its ability to prevent demineralization.
Discolorations due to fluorosis are caused by excessive use of fluoride during development of enamel [19]. High levels of fluoride in drinking water, toothpastes, nutritional supplements and dental materials are risk factors for fluorosis, particularly in children under 8 years of age [20].
It has been reported that the best results in fluoride administration can be taken from daily use of low concentration agents. Although enamel may dissolve in low pH levels, presence of low concentration of fluoride provides the dissolved minerals to participate in remineralization cycle and prevent mineral loss [21]. There is a critical pH level for fluoride to be effective in formation of fluorapatite. The pH levels between which fluoride works more effectively are reported as 4.5-6. The working mechanism of fluoride in accordance with pH level can be explained by the Stephan Curve (Figure 2).
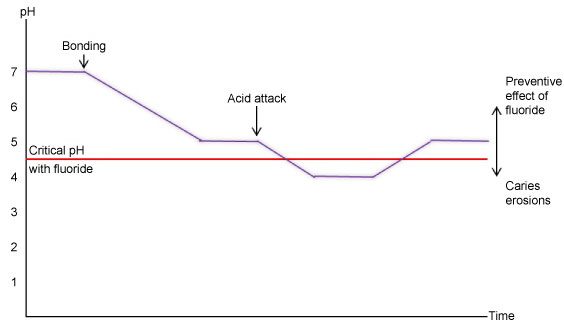 Figure 2: Stephan curve showing the working mechanism of fluoride in accordance with pH level.
View Figure 2
Figure 2: Stephan curve showing the working mechanism of fluoride in accordance with pH level.
View Figure 2
Harper, et al. [22] compared the anticariogenic potentials of four different type of cheese, each containing different levels of fat, protein, calcium and phosphate. It was found that cheese with the most protective properties against caries formation was the one that involves the greatest amount of casein phosphoproteins and calcium phosphate in its content.
Acid casein, which is a protein commonly found in mammalian milk, can reduce formation of caries when added into toothpastes. However, the amount of casein required makes any toothpaste unusable because of its taste. Sodium caseinate added into chocolate also reduces cariogenicity, but it is stated that the amount of caseinate required (16.6%) makes the product unusable for the same reason [23].
The anticariogenic mechanism of CPP-ACP can be summarized as increasing the level of calcium phosphate in plaque, reducing demineralization and increasing remineralization of enamel [23]. There are studies showing that CPP inhibits adherence and functioning of cariogenic streptococcus bacteria in mouth [23].
Scupbach, et al. [24] showed that the adherence ability of bacteria such as Streptococcus sobrinus and Streptococcus mutans decreased considerably after administration of CPP. Reynolds, et al. [25] showed that use of CPP-ACP between 0.5-1.0% concentration is equivalent to 500 ppm of fluoride in reducing cariogenic activity. An advantage of CPP-ACP over fluoride is not causing fluorosis due to high doses. Therefore use of CPP-ACP may reduce the risk of fluorosis as it reduces the need for fluoride [26].
It was thought that remineralization capacity of fluoride ions could be increased by using calcium ions. Nevertheless, the success of remineralization obtained by calcium fluoride which was formed when fluoride and calcium ions came together was found to be inadequate [27].
Chemotherapeutic agents cannot prevent formation of dental plaque, but they can be used to remove microorganisms, which is one of the main factors that cause enamel demineralization. Chlorhexidine and benzalkonium chloride are the most preferred antimicrobial agents for this purpose [28]. There are a number of studies showing that these agents significantly reduce Streptococcus mutans levels, which are responsible for demineralization and caries formation [28,29].
Regular use of these agents twice a day causes discoloration on teeth. Therefore it has been reported that 0.2% chlorhexidine mouthwash can be used at certain intervals in addition to other protection methods during orthodontic treatment, in order to reduce its side effects [30].
It is known that xylitol shows anticariogenic effect by limiting caries lesion. This effect is based on the fact that it is an non-fermentable sugar and it inhibits proliferation and growth of Streptococcus mutans [31].
It is recommended to use xylitol in lozenge form as chewing gum is not recommended in patients receiving orthodontic treatment. However, it should be noted that xylitol affects digestive system adversely and overdose usage should be avoided [32].
Prolongation of orthodontic treatment increases the risk of white spot lesion and caries formation. Therefore, fluoride release from bonding systems around brackets can be useful [10].
Sealants are surface shields that form a protective barrier against acid attacks. There are two types of sealants as chemical and light curing. Chemical-curing sealants were used initially, however disadvantages such as uncured layers, have arisen over time [33]. Studies showed that light-curing sealants are better polymerized and have a better barrier for demineralization as they cover surface completely [33].
Glass ionomer cement is a material that has desired properties such as fluoride release and chemical bonding. However, bonding with glass ionomer increases plaque accumulation around the brackets [3]. Additionally, it is not used for bonding brackets due to low bonding strength. Resin-modified glass ionomer cements were developed to overcome these side effects by adding resin particles in the cement. This adhesive system releases fluoride like conventional glass ionomer cements and has a higher adhesion force [34].
It has been thought that fluoride should be added into adhesives for continuous fluoride release, in order to prevent white spot lesion formation during orthodontic treatment without patient co-operation. It was reported that fluoride releasing adhesives provided protection for an area of 1 mm around brackets, whereas fluoride-free adhesives cannot prevent demineralization around and under brackets [12].
Topical fluoride application on lesions is considered as the first step of white spot lesion treatment. Application of fluoride in high concentration following completion of orthodontic treatment provides remineralization on lesion surfaces. However untreated parts may remain in deeper layers of lesions and continue to create aesthetic problems. Therefore, penetration of low doses of calcium and fluoride from saliva should be allowed after orthodontic treatment to obtain more aesthetic results [3]. Acid etching followed by fluoride application or acid involving fluoride application facilitates remineralization [17].
Bleaching can be applied to camouflage remaining white spot lesions, following natural remineralization which occurs by itself without any intervention. This method can be applied overnight at home or by a professional in dental office by using gel bleaching systems involving different dosages of hydrogen peroxide with the help of transparent trays in patients suffering from yellowish discoloration [35].
Knösel, et al. [36] examined the effect of bleaching on inactive white spot lesions and intact enamel surrounding them following orthodontic treatment. They observed a distinct color change in both white spot lesion area and intact enamel. It was reported that areas with white spot lesions were camouflaged since greater amount of whitening occurred in intact enamel area.
Reynolds and Black [23] found that CPP-ACP obtained from milk affected caries development. Free calcium and phosphate ions in CPP-ACP can be easily transferred to enamel surface. CPP-ACP agents are produced in various ways such as foam, mouthwash, topical paste, chewing gum and unsweetened lozenge form to transport calcium and phosphate.
Reynolds [37] reported that using a solution containing 1% CPP-ACP increased calcium level by 144% and phosphate level by 160% in oral environment, which led to decrease in mineral loss by 51% due to consumption of sugary solution. As a result, the anti-caries characteristic of CPP-ACP was demonstrated.
CPP-ACP increases amount of calcium and phosphate ions above the critical level required for remineralization. Thus, it exhibits anticariogenic effect [38]. Increased calcium and phosphate levels in plaque increase pH level of environment. Thus, demineralization is reduced and remineralization is increased. In a study, a solution containing 1% CPP-ACP was shown to treat white spot lesions 55% more compared to a water-only solution [25].
Microabrasion is a method based on controlled removal of enamel surface by applying different mixtures [39]. As the outer surface of enamel is rich in fluoride and more resistant to external factors, less enamel can be removed in the first step of microabrasion.
The most common method of microabrasion to remove white spot lesions is polishing labial/buccal surface of teeth with a rotating device by using a gel formed mixture of 18% hydrochloric acid (HCl) and medium grained pumice [40].
Microabrasion process removes some amount of enamel from tooth surface and forms a smoother enamel texture. Calcium and phosphate minerals seals interprismatic spaces as a result of microabrasion and enamel surface becomes more resistant to external factors [41].
Studies showed that application of fissure sealants to occlusal surfaces prevents caries formation. This fact led clinicians to use of flowable resins for treatment of initial enamel caries [42]. Histological studies showed that microporosity increases in different layers of early enamel lesions. It is known that these porous spaces can absorb liquids such as water like a sponge. In addition, these small porous openings and enlarged intercrystalline spaces act like diffusion paths for acids and dissolved minerals. Filling these porous spaces with low-viscosity resins, instead of completely removing newly emerging lesions, reduces the microporous structure of enamel and supports dental tissue mechanically [43].
Unlike fissure sealant technique, resins applied in caries filling technique do not cover the initial enamel caries like a hat; they rather create a barrier in the lesion by capillary movement. Thus, dental tissues are supported mechanically, whereas fracture and cavitation of surface enamel is prevented. The high penetration value of resin fillers allows enamel lesion to act like a sponge by absorbing the resin and filling pores. It is accepted that preventing spread of organic acids in an enamel lesion prevents progress of decay [44].
Infiltrants are light-curing resin materials developed in a structure that can easily penetrate into the capillary configuration of enamel lesions. The viscosity of these materials is low, their contact angles to enamel are narrow and their surface tensions are high [45].
The basic principle of the resin infiltration technique is; inhibition of lesion progression by blockage of micropores that provide a diffusion pathway for acids by using resin [46]. It is suggested that bacteria stuck under the uppermost layer may trigger decay process again despite resin blockage. However some authors suggested that these bacteria are not harmful if the porous openings are sealed properly [47].
Based on the results of in-vivo and in-vitro studies examining the efficacy low-viscosity resins used for filling caries lesions by infiltration, the material named ICON (DMG Chemisch-Pharmazeutische, Hamburg, Germany) has begun to be used. ICON is used effectively in treatment of white spot lesions [42,43].
Invasive therapies are rarely preferred in early enamel lesions that are not cavitated enough to be visible. On the other hand, resin infiltrants can only fill microcavities. Hence, they are not appropriate for treatment of lesions exceeding the superficial 1/3 of dentin and that have visible cavitation. Progression of enamel lesions is recommended to be stopped at an early stage and resin infiltration technique is an effective method as it slows or prevents the progression of lesions [48].
The exclusion criteria used in this review was not very strict and allowed inclusion of studies having mixed-method study designs. This situation might have caused contradictions in interpretation of some study outcomes and is the limitation of this review.
Oral hygiene motivation, topical fluoride agents, casein phosphopeptide-amorf calcium phosphate agents, antimicrobial agents, tooth bleaching, microabrasion and resin infiltration are the current options for prevention and treatment of white enamel lesions. Reducing the risk of lesion formation by using these methods and early treatment with the appropriate technique is recommended to obtain healthier and more aesthetic results. Future innovations in this field may bring up more treatment options of white spot lesions.