International Journal of Oral and Dental Health
Oral Health, Dysphagia, Distress, and Health Service Needs of Head and Neck Cancer Survivors 5 Years Post-Chemoradiotherapy
Bena Cartmill*, Elizabeth Ward, Olivia MacGinley and Sandro Porceddu
Centre for Functioning and Health Research and Princess Alexandra Hospital, Brisbane, Queensland, Australia
*Corresponding author: Bena Cartmill, Health Research Fellow and Advanced Speech Pathologist (Oncology), Centre for Functioning and Health Research and Princess Alexandra Hospital, Brisbane, Queensland, Australia, Tel: +61 403 160 913, E-mail: Bena.cartmill@health.qld.gov.au
Int J Oral Dent Health, IJODH-2-024, (Volume 2, Issue 1), Research Article; ISSN: 2469-5734
Received: December 22, 2015 | Accepted: February 08, 2016 | Published: February 11, 2016
Citation: Cartmill B, Ward E, MacGinley O, Porceddu S (2016) Oral Health, Dysphagia, Distress, and Health Service Needs of Head and Neck Cancer Survivors 5 Years Post-Chemoradiotherapy. Int J Oral Dent Health 2:024. 10.23937/2469-5734/1510024
Copyright: © 2016 Cartmill B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Purpose: Evidence suggests that oral health effects and dysphagia remain chronic conditions for patients who undergo radiotherapy with or without chemotherapy ([chemo]RT) following diagnosis of head and neck cancer (HNC), however, there is limited outcome data beyond 1-2 years post-treatment. The aim of the current study was to investigate the long term patient outcomes at 5-6 years post- (chemo)RT using patient-reported functional measures. A secondary aim was to examine the extent of services accessed, and desired, by this group.
Methods: A retrospective audit was conducted to select patients treated curatively for HNC using (C)RT and seen by speech pathology. Twenty eligible long term HNC patients treated with (chemo)RT completed a series of patient-reported outcome measures, a quality of life (QoL) scale, a general distress tool and questions relating to services.
Results: Results revealed that at 5-6 years post-treatment, over half reported moderate to severe oral health effects, and only 30% tolerated a full normal diet. Moderate to severe levels of distress were reported by 25%, though global QoL remained positive. Few had sought further services for these issues.
Conclusions: Persistent oral health effects, chronic swallowing difficulties, and distress are common in this population. Clinicians need to be aware of the long-term nature of patient-reported dysfunction and tailor appropriate services and supports to patient need.
Keywords
Head and neck cancer, Chemoradiotherapy, Survivorship, Dysphagia, Late effects, Patient reported outcomes
Introduction
Nonsurgical approaches to head and neck cancer (HNC) treatment, including radiotherapy with or without chemotherapy (chemo)RT, can significantly affect the swallowing function of patients [1-4]. Although evidence supports that many individuals will experience improved swallowing in the months following (chemo)RT, for a considerable proportion of individuals, dysphagia continues to be a persistent issue at one year post-treatment [1,3,5-7]. Furthermore, in the limited studies conducted to date, evidence suggests that dysphagia may continue to persist for many years post-treatment and that a subset may even undergo further functional decline [5,8-10].
With increasing numbers of patients living longer following cancer treatment [11], it has become important that the extent and long-term impact of nonsurgical treatment on swallow function is better understood. Many patients who have dysphagia post-treatment will continue to experience persistent swallowing difficulties a number of years later, and in some cases, present with worsening of the condition, thought to be due to the ongoing effects of tissue fibrosis causing continued functional tissue loss, leading in some instances to stiffening and hardening of tissues, and possible stricture formation [11,12]. The high prevalence of long term xerostomia [13] and dysgeusia [14] also contributes to ongoing patient-reported swallowing dysfunction due to resultant discomfort and changes to diet choices [15]. Radiation induced neuropathy and muscle atrophy are also potential causative factors for long term dysphagia and trismus, as are mucosal sensory changes following radiotherapy [11,16,17].
Existing evidence regarding long term swallowing outcomes, however, comes from only a limited number of studies. Several have focused primarily on the physiological and clinician-rated changes to swallowing function [7,8,10,18,19], and have found that the majority of patients have been unable to resume a normal diet, and present with physiological impairment [7] characterized by deficits in laryngeal movement, epiglottic deflection, tongue base retraction and pharyngeal contraction in the years following treatment [10,18,19]. Cancer survivorship literature now places greater emphasis on exploring the long-term impact of oral health effects using patient perceptions of their functional state. As such, there has been growing importance placed on using patient-reported outcome (PRO) measures, rather than clinician directed tools or physiological function measures, to explore the impact of cancer and its treatment. The few studies that have explored long term outcomes using PRO tools [20-22] also document the continuing presence of dysphagia and oral health effects more than a year following treatment, as well as the potential for a negative change in function over time. Patients have reported significant worsening of dysphagia and xerostomia to 2 years post-treatment, with lower proportions of patients able to tolerate a full diet at each follow up [5]. Swallowing dysfunction has been identified as the primary factor impacting on quality of life in HNC survivors in the year’s post-treatment [23-25], and elevated levels of depression and anxiety are significantly worse in dysphagic HNC survivors than non-dysphagics [23].
Whilst the available evidence would suggest dysphagia continues to be an issue and may increase in severity for a proportion of patients following nonsurgical treatment for HNC, the current evidence on long term outcomes is limited due to the overall small volume of studies conducted to date, studies providing valid assessment of functional change over time (e.g. lack of pre- and post- treatment data), and the lack of comprehensive examination of swallowing from the patient perspective. Furthermore, in the studies already completed, there has been no discussion of services sought by patients experiencing dysphagia in the long term following treatment. Of the two existing international studies to have reported service patterns for patients with HNC, neither provided extensive information on the provision of swallowing management long-term post-treatment [26,27]. Within Australia, the availability and uptake of services for patients with long term dysphagia is unknown. Clinical experience though, would suggest there is a potential absence of services and support mechanisms for patients presenting with long term dysphagia and associated oral health effects. Hence the primary aim of this study was to explore the incidence and nature of long term patient-reported dysphagia, oral health effects, and co-occurring distress following non-surgical treatment for HNC. The secondary aim was to determine the nature and extent of the services sought, and provided for this population.
Methods
Participants
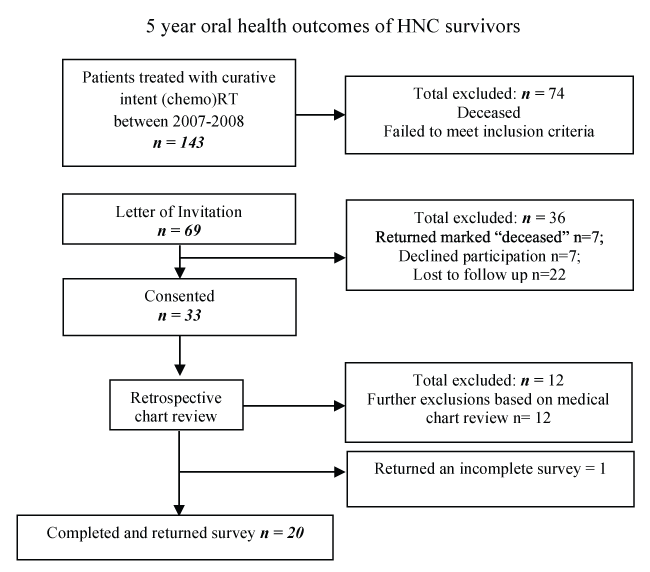
Suitable participants for this study were identified using the patient record system of the Princess Alexandra Hospital (PAH) Radiation Oncology Department in Queensland, Australia. Informed consent was obtained from all individual participants included in the study. For inclusion, participants had to have undergone (chemo)RT for HNC with curative intent 5-6 years prior to contact (between January of 2007 to December of 2008). Patients were excluded if they had: undergone palliative treatment or treatment for recurrent HNC, did not receive speech pathology intervention during or following treatment, had any pre-existing medical condition which could have impacted on swallow function, or if they had undergone primary surgical management for their HNC (excluding biopsies, isolated neck dissection or tracheostomy insertion for airway management). Figure 1 outlines the recruitment process. The details of the 20 participants can be found in table 1. The final cohort consisted of 17 males and three females with a mean age of 56.9 years (SD = 9.17) who had received 3D conformal (chemo)RT treatment 5-6 years prior to participating in the current study.
![]()
Table 1: Demographic Details of the Cohort at Presentation.
View Table 1
Procedure
All eligible participants were contacted for consent through a letter of invitation, with a follow-up phone call to determine their desire to participate. Once consented, the research team collected retrospective data from the medical chart regarding each participants swallowing and speech pathology service history for comparison with current status at 5-6 years post-treatment. Using the speech pathology entries and diet recommendations in the medical chart, the patient’s level of swallowing function at three prior time points was collected: (a) at commencement of treatment, (b) at week 4-5 of treatment, and (c) on completion of treatment (week 7-8). Functional oral intake at these time points was recorded using the Functional Oral Intake Scale (FOIS; [28] which is a 7 point scale used to describe functional swallowing outcomes and regular dietary intake of patients, where 7 is a normal diet and scores of 3 and below represent need for non-oral nutritional support. In addition, information was collected from the medical chart regarding the timing and extent of speech pathology services accessed. This information included data related to the timing and number of sessions with speech pathologists after treatment.
As part of the prospective data collection, all eligible, consenting participants were contacted and asked to complete a “self-reported” Functional Oral Intake Scale (srFOIS). Although the FOIS tool is typically completed by a clinician, it was adapted for the purposes of this study to be used as a patient-reported tool. The clinician-rated items from 0 to 7 were presented in simplified language and expressed in the first person (Appendix 1) to create the srFOIS. The scale remained the same as the FOIS, with lower numbers representing increased oral intake restrictions.
Participants also completed three additional tools at the time of contact, including: (a) the Vanderbilt Head and Neck Symptom Survey version 2.0 (VHNSS v2.0), a reliable and valid HNC specific questionnaire containing 50 items scored from 0 (no symptoms) to 10 (severe symptoms) relating to functional swallowing status and oral health effects [29], (b) the Functional Assessment of Cancer Therapy – Head and Neck (FACT H&N), a general QoL tool validated with the HNC population [30] containing four domains of functioning (physical, social/family, emotional and functional), where higher scores represent improved QoL, and (c) the Distress Thermometer a validated, patient-rated score describing stress levels in the cancer population which is scored from 0 (no distress) to 10 (extreme distress) with additional questions regarding potential reasons for distress across six areas including practical, physical, spiritual, family, emotional and other causes [31]. The battery of assessments was specifically selected to provide information on functional oral intake/dysphagia, while also indicating patient reported oral health and distress.
Patients were also asked to report on the type and extent of services accessed, and desired, through three additional multiple choice questions (Appendix 2). These questions were related to the patients’: (1) current access to health services; (2) desire for further access; and (3) goals of these services. Both questions (1) and (2) had 14 health related services provided as optional responses, with an additional option of “other” services. For question 3, participants were provided with responses regarding what they may wish to gain from accessing services.
Data was collected through various methods to optimize participation. As selected by each participant, the assessment tools and additional questions were completed either (a) in person with support from a research team member, (b) over the phone with the assistance of a researcher, (c) independently by completing hardcopy versions provided via the mail, or (d) independently via electronic versions of the tools delivered via a secure online survey site (https://www.surveymonkey.com/).
Analysis
Data collected from the aforementioned assessment tools was entered into excel and basic descriptive data for the cohort was computed. Qualitative data yielded from any free text responses provided by participants regarding services was analysed using content analysis. This was completed by one member of the research team and validated by a second. Any disagreement was resolved with discussion with a third team member. Statistical comparisons using Friedman’s tests with post hoc Wilcoxan tests were used to explore change across the FOIS data collected before, during and at the end of treatment. As the srFOIS data was collected through patient report as opposed to clinician rated, this data was analysed separately and compared to the clinician’s FOIS ratings at pre- and post- treatment using planned contrasts (Wilcoxon Signed Ranks tests). Significance was set at p < 0.05. To explore any relationships between current swallowing function, extent of long term swallowing related treatment side effects, distress and the need for services, key data obtained from the srFOIS, the VHNSS v2.0 and service data was triangulated and examined for individual patient patterns.
Results
Functional swallowing status (FOIS)
Results of the FOIS scores collected from pre-treatment, week 5 of treatment, and post-treatment revealed a significant (χ = 25.423, p = < 0.001) change in swallow functioning across time (Table 2). Post hoc analysis revealed a significant (Z = -3.742, p = < 0.005) decline in swallow function from pre-treatment to week 5 of treatment and also from pre-treatment to post-treatment (Z = -3.528, p = < 0.005). There was no significant (p > 0.05) difference observed between FOIS scores between week 5 and the end of treatment. Overall descriptive statistics revealed the majority (80%) of patients were tolerating a normal diet prior to treatment which dropped to less than 20% at week 5 and at immediately post-treatment (Table 2). One participant in the study cohort required alternative feeding prior to commencing treatment (Functional Oral Intake Scale score of 1-3), which increased to 4 participants in the early post-treatment phase.
![]()
Table 2: Functional Swallowing Ability (FOIS) and Self-Reported Functional Swallow Ability (srFOIS) Relative to Treatment (n = 20).
View Table 2
Using planned contrasts, a significant (Z = 3.596, p = < 0.005) improvement in functional swallow status was observed between the participants FOIS score reported post-treatment, and their current srFOIS at 5-6 years later. Descriptive statistics revealed that 30% had returned to a full normal diet, while the majority (60%) reported they were now tolerating a non-texture modified diet but still must avoid specific foods or liquid items (srFOIS level 6). At 5-6 years post-treatment, only 10% required a texture modified diet (Table 2), compared to immediately following treatment where 45% were on modified texture diets (FOIS level 5). By 5 years post treatment, no participants required alternative feeding. Despite this improvement, at 5-6 years post-treatment the mean srFOIS scores remained significantly (Z = -2.500, p = 0.012) lower than pre-treatment. At pre-treatment, the large majority (80%) of patients were managing a full, normal diet, while only 30% had returned to this level at 5-6 years post-treatment.
Patient-reported outcomes
Results of the VHNSS v2.0 revealed that all participants were reporting having some negative health outcomes (toxicity rating ≥ 1) in each of the main symptom categories at 5-6 years post-treatment (Table 3). Further examination revealed that > 25% of the current cohort reported moderate to severe difficulties (toxicity scores ≥ 4) within all domains except nutrition, mucositis, smell and pain (Table 3). In terms of specific dysphagia related items, moderate to severe difficulties in eating solids, xerostomia, increased eating duration, sensitivity to dryness and sensitivity to spicy, hot or acidic foods were reported by more than half of the cohort.
![]()
Table 3: Frequency and Severity of Oral Health Outcomes on the VHNSS v2.0.
View Table 3
When analysed in relation to parameters described by List et al. [30], the QoL of the participants as determined by the FACT H&N (Table 4) was better than average. List et al. [30] classified QoL scores into those reported by patients with ‘good’ or ‘poor’ overall performance as rated by a Karnofsky scale. The mean QoL score indicated by the current cohort in each domain is better than the average score reported by patients with ‘good’ global functioning with the exception of social wellbeing. The mean social wellbeing score was slightly below the mean score reported by List et al. [30].
![]()
Table 4: Patient Reported Long term Quality of Life Following Treatment Using the FACT-H&Na
View Table 4
Distress
Data from the Distress Thermometer tool revealed that 45% of participants were experiencing some degree of ongoing general distress (scores > 0) at 5-6 years post-treatment, with 25% of patients’ reporting moderate to severe (> 4) levels of distress. Main causes of distress reported by the cohort are reported in table 5. Fatigue was a source of distress for 40% of the cohort, while 30% of the participants reported eating and drinking difficulties as a cause.
![]()
Table 5: Most Common (≥ 25%) Causes of Patient Rated Distress on Distress Thermometer.
View Table 5
Services
Review of patient medical records revealed that 60% of patients accessed speech pathology services following completion of (chemo)RT, of which an average of three sessions were attended. The majority of these services were provided in the initial six weeks after (chemo)RT. Only one participant was reported to have received speech pathology services beyond 12 weeks following treatment completion. Regarding services participants were currently accessing, 50% remained involved with health professionals, although this was largely contact with medical professionals (40% otolaryngologist; 25% general practitioner, and 15% radiation oncologist). Only 5% continued to visit a speech pathologist 5-6 years following treatment. The majority of participants reported limited desire for further services beyond those already being sought, with only two patients expressing interest in receiving further support from any health professionals.
Data triangulation
Comparison of the data obtained from the dysphagia related items of the VHNSS v2.0, the distress scale and the services data revealed that there was some relationship between these key data points (Table 6). The participants with increased functional swallowing deficits (srFOIS ≤ 6) on average reported more moderate to severe swallowing related side effects on the VHNSS than those tolerating a normal diet (srFOIS = 7). There were also no participants tolerating a non-texture modified diet (srFOIS = 7) who reported moderate to severe distress levels. However, desire for services did not appear to be related to high distress, the lowest srFOIS scores or the most significant levels of dysphagia related side effects.
![]()
Table 6: Data Triangulation of Swallowing, Oral Health, Distress and Service Information.
View Table 6
Discussion
The results of this study indicate that the majority of patients report ongoing dysphagia 5-6 years following (chemo)RT for HNC. Furthermore all patients were reporting ongoing negative oral health. Distress was an issue for almost half of the individuals. Despite these multifaceted physical and emotional issues, few additional services were desired or being sought by patients to assist in their management. The current data supports previous studies in which swallowing difficulties, psychological distress and continuing side effects have been reported by patients’ long term following nonsurgical HNC treatment [5,21,22,32] and contributes important new insights into long term service needs.
Comparison of the FOIS data collected from before and immediately following treatment, confirmed that the majority of the current cohort experienced significant dysphagia during, and at the end of (chemo)RT treatment. Comparison of that data with patient reported swallowing status at 5-6 years post-treatment revealed that significant functional improvements had been experienced. With respect to the severity of dysphagic symptoms, only 10% of the current cohort continued to require texture modified diets, compared to 33% in the Frowen et al. [8] cohort at 5-6 years post-treatment. However, swallowing function had not returned to pre-treatment levels, with only 30% of the cohort able to manage a full unrestricted diet at long term follow up. This incidence is lower than that reported by Cartmill et al. [5], Frowen et al. [8], and Newman et al. [33], who found that 42%, 59%, and 72% of their cohorts, respectively, had returned to normal diets more than a year following radiotherapy or chemoradiotherapy. Conversely, Berg et al. [7] found that no patients in their cohort of 32 were tolerating a normal diet at 14-68 months post-chemoradiotherapy. The current findings support the growing body of literature which indicate that swallowing difficulties remain a chronic condition for a proportion of patients in the long term following HNC management [5,6,8,9,11].
Patient report revealed that the nature of the difficulties experienced were largely relating to difficulties eating solids and an increased eating duration. A proportion (≥ 30%) of the participants also indicated moderate to severe symptoms of coughing after swallowing, reported getting food stuck in the throat and mouth, and experienced choking on solids. Similar results were reported for the heterogenous cohort of patients 6-166 months post- HNC treatment studied by Cooperstein et al. [29]. It is possible that these specific swallowing difficulties/symptoms (coughing, food sticking, choking) reported by participants in the current study relate to the presence of pharyngeal residue post swallow and subsequent post swallow penetration/aspiration. Such physiological difficulties have previously been reported as ongoing issues following nonsurgical HNC treatment [8,10,19].
The current cohort also reported ongoing negative oral health alongside their dysphagia. Almost all of the participants reported experiencing xerostomia, with the majority having moderate to severe issues affecting their ability to sleep, talk and chew. Increased mucosal sensitivity in relation to dryness and spicy, acidic and hot foods were reported by the majority of patients, as well as altered taste and thick mucus. Oral health difficulties in relation to mucosal sensitivity and taste change resulted in patients altering their diet choices. Mucosal sensitivity, causing eating and drinking issues, has been well recognized in prior studies [15,34]. Previous studies have similarly reported continued incidences of xerostomia, taste changes, swallowing difficulties, limited range of movement and altered mucus production many years after treatment [5,21,22,35]. The significant ongoing issues relating to xerostomia and mucosal sensitivity reported by the current cohort of HNC patients adds to the growing body of evidence supporting the presence of multiple long term difficulties contributing to functional deficits of oral intake.
Recent qualitative research by Nund et al. [36] found that patients may experience ongoing distress associated with dysphagia following non-surgical treatment. Indeed, distress was found to be a continuing issue in the current cohort, with a quarter reporting distress levels of 4 or greater on the Distress Thermometer, an indicator of clinically significant distress [37]. This finding is comparable to previous reports of a 27% distress rate in patients up to 10 years following treatment (surgical or nonsurgical) [38]. In the current cohort, 30% of participants identified eating and drinking as a cause of their ongoing distress. Bjordal and Kaasa [32] similarly found that 64% of patients with high levels of swallowing difficulties had high levels of distress, while half of those experiencing dry mouth, taste problems, coughing and difficulties related to mucus production were also classified as clinically distressed. The long term distress levels reported in both the current and previous studies indicate that despite extended periods of time following treatment, a patient’s psychological adaptation to their dysphagia is not ensured.
Interestingly, despite reporting a degree of ongoing distress many years after treatment, global QoL of the current cohort was found to be positive. It was found that mean scores in each domain (except social wellbeing) were higher than those provided by patients rated as having good global performance scores by List et al. (1996). Good QoL, even in the presence of ongoing treatment related deficits beyond 1 year after nonsurgical treatment, is a recurring trend in the literature exploring patient-reported outcomes [21,35,39]. de Graeff et al. [35] proposed that possible reasons for this discrepancy are patient adaptation causing a possible response shift, with less fear of recurrence/death many years after treatment. These reasons may also be proposed to account for the current positive QoL findings.
All the patients included in this study accessed speech pathology services during their nonsurgical treatment, with the majority receiving intervention in the first 12 weeks following completion. However beyond this acute phase, only 1 patient continued to receive speech pathology services. Little is known regarding post-discharge speech pathology services for this population, as the only two studies [26,27] which have examined services provided to HNC patients have failed to provide details regarding ongoing post-treatment intervention. The information obtained from the participants in the current study revealed that although half continued to receive ongoing services from medical professionals (Ear, Nose and Throat specialists and general practitioners), very few reported receiving any allied health services, which would suggest that minimal ongoing engagement with rehabilitation services was undertaken in the long term.
Furthermore, it was found that only a small number of participants wish for further services despite ongoing dysphagia, negative oral health effects and distress. The lack of interest in further intervention could be related to the positive QoL reported by the cohort, whereby patients acknowledge their ongoing difficulties, but retain a positive QoL and therefore do not seek any further additional intervention in the long term. This argument is consistent with a degree of adaption to their difficulties years after treatment [35,36]. Equally though, it is possible that participants may be unaware of any benefits which could be provided from further intervention, and as such, they fail to seek services. It is important that individuals are made aware of possible services which could assist them, so they can make informed choice about seeking out any further supports long term post-treatment.
Using triangulation of key data obtained relating to ongoing oral health effects, distress and desire for services, a relationship was revealed between increased side effects and decreased swallowing function. There was also increased distress found in those not tolerating a normal diet. Interestingly though, a desire for services was not indicated by the patients with the most severe ongoing side effects, distress levels or swallowing difficulties. This may indicate that patients have adapted and accepted their current level of difficulty with no desire for intervention, as discussed previously. Or it may also suggest that they may be unaware of what services are available, or how to access these services to aid their swallowing, psychological and general wellbeing. It is also possible that participants were considering only those services which targeted supportive intervention for physical impairments. Considering the levels of distress and long term negative changes to the health state which persist post- (chemo)RT, it may be that services which support patients’ psychosocial needs may be most appropriate for them at this stage of their cancer survivorship journey.
Although the current study has provided further validation of the extent and nature of long term swallowing and treatment related changes for HNC patients 5-6 years following (chemo)RT, there are a number of limitations that must be acknowledged. Although the focus of this research was specifically to explore the patients’ perspective of their current functioning, the addition of a clinical assessment of swallowing status in future studies could provide greater insight into the physiological factors contributing to the swallowing difficulties being reported. Equally, including a qualitative component to explore the issue of services, service needs and overall awareness of services available, would have provided more insight into the needs of this population and their awareness of the services available. A further limitation of the current study was the small cohort numbers. Although recruitment included a 2 year treatment window, exclusion criteria, mortality and difficulties contacting participants 5-6 years after treatment resulted in significant attrition of the sample size, which is a natural consequence of the population being studied, and the authors acknowledge a potential response bias which such attrition rates. However, future studies which recruit from multiple sites could allow patterns to be examined in larger, more representative cohorts. Consistent with the nature of HNC and its management, it is also recognized that the cohort was not homogeneous, with participants representing a range of cancer stages and tumor locations, and having undergone differing modes of non-surgical treatment. Additionally, no consistent reporting of HPV status was available for this cohort. Unfortunately due to the small cohort size, no sub-analysis of outcomes by tumor demographics is possible. Future studies with larger cohorts that could potentially stratify by cancer site, stage and treatment modality may find differential patterns regarding the extent and severity of long term outcomes in certain subpopulations. This warrants further investigation so that patients can be more fully informed regarding potential long term outcomes following different treatments.
Conclusion
The current study adds to the body of literature investigating the continuing presence of dysphagia in the long term following (chemo)RT. Ongoing patient-reported swallowing difficulties and oral health effects, including xerostomia, were highlighted 5-6 years post-treatment. Distress was a continuing factor in the population, with at least one third attributing this to their eating and drinking issues. Despite this, patient-reported global QoL was good. This positive QoL was potentially reflected in the small number of patients requesting the need for support services related to their HNC care. Despite participants indicating little interest in seeking further services, the fact that the current data confirms the presence of long term dysphagia, persistent negative oral health effects, and ongoing distress, highlights the need to ensure support structures are in place and available for patients beyond the acute care stage. Further studies into this issue are needed to determine the nature of services that may be of most benefit for patients in the survivorship phase.
Conflict of Interest
The authors have no conflict of interest to disclose.
Statement of Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Princess Alexandra Hospital and Metro South Human Research and Ethics Committee (HREC/13/QPAH/315) and with the 1964 Helsinki declaration and its later amendments.
References
-
Wall LR, Ward EC, Cartmill B, Hill AJ (2013) Physiological changes to the swallowing mechanism following (chemo) radiotherapy for head and neck cancer: a systematic review. Dysphagia 28: 481-493.
-
Shune SE, Karnell LH, Karnell MP, Van Daele DJ, Funk GF (2012) Association between severity of dysphagia and survival in patients with head and neck cancer. Head Neck 34: 776-784.
-
Hutcheson KA, Lewin JS, Barringer DA, Lisec A, Gunn GB, et al. (2012) Late dysphagia after radiotherapy-based treatment of head and neck cancer. Cancer 118: 5793-5799.
-
Murphy BA, Gilbert J (2009) Dysphagia in head and neck cancer patients treated with radiation: assessment, sequelae, and rehabilitation. Semin Radiat Oncol 19: 35-42.
-
Cartmill B, Cornwell P, Ward E, Davidson W, Porceddu S (2012) Long-term functional outcomes and patient perspective following altered fractionation radiotherapy with concomitant boost for oropharyngeal cancer. Dysphagia 27: 481-490.
-
Szczesniak MM, Maclean J, Zhang T, Graham PH, Cook IJ (2014) Persistent dysphagia after head and neck radiotherapy: a common and under-reported complication with significant effect on non-cancer-related mortality. Clin Oncol 26: 697-703.
-
van den Berg MG, Rütten H, Rasmussen-Conrad EL, Knuijt S, Takes RP, et al. (2014) Nutritional status, food intake, and dysphagia in long-term survivors with head and neck cancer treated with chemoradiotherapy: a cross-sectional study. Head Neck 36: 60-65.
-
Frowen J, Drosdowsky A, Perry A, Corry J (2014) Long-term swallowing after chemoradiotherapy: Prospective study of functional and patient-reported changes over time. Head Neck .
-
Kraaijenga S, Oskam I, van der Molen, Hamming-Vrieze, Hilgers F, et al. (2015) Evaluation of long term (10-years+) dysphagia and trismus in patients treated with concurrent chemo-radiotherapy for advanced head and neck cancer. Oral Oncol 51: 787-794.
-
Smith RV, Kotz T, Beitler JJ, Wadler S (2000) Long-term swallowing problems after organ preservation therapy with concomitant radiation therapy and intravenous hydroxyurea: initial results. Arch Otolaryngol Head Neck Surg 126: 384-389.
-
Hutcheson KA (2013) Late radiation-associated dysphagia (RAD) in head and neck cancer survivors. Perspectives on Swallowing and Swallowing Disorders (Dysphagia) 22: 61-72.
-
Wynn TA (2008) Cellular and molecular mechanisms of fibrosis. J Pathol 214: 199-210.
-
Deboni AL, Giordani AJ, Lopes NN, Dias RS, Segreto RA, et al. (2012) Long-term oral effects in patients treated with radiochemotherapy for head and neck cancer. Support Care Cancer 20: 2903-2911.
-
Epstein JB, Emerton S, Kolbinson DA, Le ND, Phillips N, et al. (1999) Quality of life and oral function following radiotherapy for head and neck cancer. Head Neck 21: 1-11.
-
Logemann JA, Pauloski BR, Rademaker AW, Lazarus CL, Mittal B, et al. (2003) Xerostomia: 12-month changes in saliva production and its relationship to perception and performance of swallow function, oral intake, and diet after chemoradiation. Head Neck 25: 432-437.
-
Chen AM, Hall WH, Li J, Beckett L, Farwell DG, et al. (2012) Brachial plexus-associated neuropathy after high-dose radiation therapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys 84: 165-169.
-
Manikantan K, Khode S, Sayed SI, Roe J, Nutting CM, et al. (2009) Dysphagia in head and neck cancer. Cancer Treat Rev 35: 724-732.
-
Graner D, Foote R, Krein K (2009) Serial swallow studies over seven years with three patients treated with chemoradiation for head and neck cancer. Dysphagia 24: 476.
-
Hutcheson KA, Lewin JS (2012) Functional outcomes after chemoradiotherapy of laryngeal and pharyngeal cancers. Curr Oncol Rep 14: 158-165.
-
Bjordal K, Freng A, Thorvik J, Kaasa S (1995) Patient self-reported and clinician-rated quality of life in head and neck cancer patients: A cross-sectional study. Eur J Cancer Oral Oncol 31: 235-241.
-
Hammerlid E, Taft C (2001) Health-related quality of life in long-term head and neck cancer survivors: a comparison with general population norms. Br J Cancer 84: 149-156.
-
Magné N, Marcy PY, Chamorey E, Guardiola E, Pivot X, et al. (2001) Concomitant twice-a-day radiotherapy and chemotherapy in unresectable head and neck cancer patients: A long-term quality of life analysis. Head Neck 23: 678-682.
-
García-Peris P, Parón L, Velasco C, de la Cuerda C, Camblor M, et al. (2007) Long-term prevalence of oropharyngeal dysphagia in head and neck cancer patients: Impact on quality of life. Clin Nutr 26: 710-717.
-
Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, Leemans CR, Aaronson NK, et al. (2008) Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol 26: 3770-3776.
-
Nguyen NP, Frank C, Moltz CC, Vos P, Smith HJ, et al. (2005) Impact of dysphagia on quality of life after treatment of head-and-neck cancer. Int J Radiat Oncol Biol Phys 61: 772-778.
-
Krisciunas GP, Sokoloff W, Stepas K, Langmore SE (2012) Survey of usual practice: dysphagia therapy in head and neck cancer patients. Dysphagia 27: 538-549.
-
Roe JW, Carding PN, Rhys-Evans PH, Newbold KL, Harrington KJ, et al. (2012) Assessment and management of dysphagia in patients with head and neck cancer who receive radiotherapy in the United Kingdom - a web-based survey. Oral Oncol 48: 343-348.
-
Crary MA, Mann GD, Groher ME (2005) Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil 86: 1516-1520.
-
Cooperstein E, Gilbert J, Epstein JB, Dietrich MS, Bond SM, et al. (2012) Vanderbilt Head and Neck Symptom Survey version 2.0: report of the development and initial testing of a subscale for assessment of oral health. Head Neck 34: 797-804.
-
List MA, D'Antonio LL, Cella DF, Siston A, Mumby P, et al. (1996) The Performance Status Scale for Head and Neck Cancer Patients and the Functional Assessment of Cancer Therapy-Head and Neck Scale. A study of utility and validity. Cancer 77: 2294-2301.
-
Roth AJ, Kornblith AB, Batel-Copel L, Peabody E, Scher HI, et al. (1998) Rapid screening for psychologic distress in men with prostate carcinoma: a pilot study. Cancer 82: 1904-1908.
-
Bjordal K, Kaasa S (1995) Psychological distress in head and neck cancer patients 7-11 years after curative treatment. Br J Cancer 71: 592-597.
-
Newman LA, Vieira F, Schwiezer V, Samant S, Murry T, et al. (1998) Eating and weight changes following chemoradiation therapy for advanced head and neck cancer. Arch Otolaryngol Head Neck Surg 124: 589-592.
-
Garden AS, Chambers MS (2007) Head and neck radiation and mucositis. Curr Opin Support Palliat Care 1: 30-34.
-
de Graeff A, de Leeuw JR, Ros WJ, Hordijk GJ, Blijham GH, et al. (2000) Long-term quality of life of patients with head and neck cancer. Laryngoscope 110: 98-106.
-
Nund RL, Ward EC, Scarinci NA, Cartmill B, Kuipers P, et al. (2013) The lived experience of dysphagia following non-surgical treatment for head and neck cancer. Int J Speech Lang Path 16: 282-289.
-
Donovan KA, Grassi L, McGinty HL, Jacobsen PB (2014) Validation of the distress thermometer worldwide: state of the science. Psychooncology 23: 241-250.
-
Verdonck-de Leeuw IM, Eerenstein SE, Van der Linden MH, Kuik DJ, de Bree R, et al. (2007) Distress in spouses and patients after treatment for head and neck cancer. Laryngoscope 117: 238-241.
-
El-Deiry M, Funk GF, Nalwa S, Karnell LH, Smith RB, et al. (2005) Long-term quality of life for surgical and nonsurgical treatment of head and neck cancer. Arch Otolaryngol Head Neck Surg 131: 879-885.