International Journal of Neurology and Neurotherapy
Delay in Pusher Syndrome Recovery is Related to Frontal White Matter Lesions
Hiroaki Abe1,2*, Takeo Kondo2,3, TakanoriKochiyama4, Yutaka Oouchida2, Satoru Fujiwara5 and Shin-Ichi Izumi2,6
1Department of Rehabilitation Medicine, Kohnan Hospital, Japan
2Department of Physical Medicine and Rehabilitation, Tohoku University, Japan
3Department of Rehabilitation Medicine, Syodokai Minamitohoku General Hospital, Japan
4Brain Activity Imaging Center, Advanced Telecommunications Research Institute, Japan
5Department of Neurosurgery, Kohnan Hospital, Japan
6Tohoku University, Graduate School of Biomedical Engineering, Japan
*Corresponding author:
Hiroaki Abe, Department of Rehabilitation Medicine, Kohnan Hospital, Nagamachi Minami 4-20-1, Taihaku-ku, Sendai, Japan, Tel: +81-248-2131, Fax: +81-248-1906, E-mail: abehi0827@gmail.com
Int J Neurol Neurother, IJNN-4-065, (Volume 4, Issue 1), Original Article; ISSN: 2378-3001
Received: November 24, 2016 | Accepted: February 10, 2017 | Published: February 13, 2017
Citation: Abe H, Kondo T,Kochiyama T, Oouchida Y, Fujiwara S, et al. (2017) Delay in Pusher Syndrome Recovery is Related to Frontal White Matter Lesions. Int J Neurol Neurother 4:065. 10.23937/2378-3001/1410065
Copyright: © 2017 Abe H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective: Unilateral stroke can lead to a disorder of postural balance that manifests as a pushing toward the paretic side, termed "pusher syndrome" (PS). The relationship between lesion location and the time course of recovery of PS is still unclear. Thus, this study investigated the relationship between the time course of PS and lesion sites.
Methods: We investigated nine patients with acute ischemic stroke in the right hemisphere of the brain. The time course of the severity of PS was assessed using the standardized Scale for Contraversive Pushing. Patients were divided into two groups: the recovery and no recovery groups. Magnetic resonance imaging data were obtained to assess the effect of ischemic lesion sites on the recovery of PS and was analyzed with lesion subtraction technique.
Results: The subtraction imaging revealed an association between delay in the recovery of PS and frontal white matter lesions. These regions corresponded to the cortico-spinal tract and superior longitudinal fasciculus.
Conclusions: Previous studies revealed that patients with PS required longer rehabilitation to reach outcome goals than patients without PS. Our results indicate that when patients with PS have right frontal white matter lesions, planning a long rehabilitation should be considered compared with patients with other lesions.
Keywords
Pusher syndrome, Stroke, Magnetic resonance imaging, Rehabilitation, Postural abnormality
Introduction
Unilateral stroke can lead to a disorder of postural balance that manifests as a pushing toward the paretic side, termed "pusher syndrome" (PS) [1]. The reported incidence of this syndrome varies from 8% to 63% in all patients with stroke [2-7]. This large variability is probably due to differences or biases in the assessment and selection procedures [6]. A clinical rating scale for evaluating the severity of PS was developed in previous studies [8-10] and is termed the Scale of Contraversive Pushing (SCP). Recently, we revealed the prevalence of PS to be 14.2% in all patients with stroke and 9.4% in stroke patients who suffered motor deficit using valid quantitative assessment in a large sample study of 1660 subjects recruited from acute inpatients [11]. Previous studies showed that patients with PS have lower functional independence measure efficacy [12] and a slower process of recovery [4]. Babyar, et al. [5] found worse outcomes for the group of patients with right hemisphere stroke. Also, patients with PS might require longer rehabilitation to reach outcome goals [12]. Previous reports indicated that PS is typically associated with lesions of the posterior thalamus [13], posterior insula, and subcortical region of post-central gyrus [14]. However, other lesions have been reported in PS [15] and multiregional lesions have been associated with PS by perfusion magnetic resonance imaging (MRI) [16].
There have been reports of good prognosis of PS [3], but the duration of PS behavior differs widely. Krewer, et al. [12] reported that the mean duration of patients with PS in a rehabilitation hospital was 5 ± 4.3 weeks. The effect worsened if PS had been present for a longer period [12]. Therefore, prognostic evaluation is very important to plan rehabilitation goals and estimate the length of intervention. Recently, a large clinical study showed that patients with right hemisphere lesions (RHL) exhibited a significantly slower recovery from PS than those with left hemisphere lesions (LHL) [11]. However, a relationship between lesion location and the time course of recovery of PS remains unclear. Thus, we investigated the relationship between the time course of PS and RHL.
Methods
Subjects
We conducted a retrospective cohort study of patients with acute stroke (infarction, hemorrhage, subarchnoid hemorrhage, and severe symptomatic stenosis) admitted to Kohnan Hospital from July 2006 to January 2009. Stroke was diagnosed based on neurological signs and brain computed tomography scans and/or MRI.
The evaluation of PS was performed according to the SCP [8-10]. The degree of PS was evaluated daily during physical therapy. Moreover, for this study, we added new inclusion criteria: 1) all patients were identified as right side unilateral ischemic stroke with MRI because PS resolved early in patients with LHL [11], and this retrospective study could not obtain early MRI in hemorrhagic stroke patients, 2) all patients with severe PS (SCP > 3), and 3) no severe hypo-perfusion in extra-lesion areas identified with single photon emission computed tomography. Because we believed a small cerebellar lesion would not affect the recovery of PS [17], patients with very small left cerebellar lesions identified with MRI were included.
This study was conducted in compliance with the ethical principles of the Declaration of Helsinki regarding biomedical research on human subjects, and informed consent was obtained. We obtained approval from our institutional ethics committee prior to study initiation.
Clinical assessment
Evaluation of PS was performed according to the SCP [8-10] on the day of the first training session for sitting and/or standing. We used conventional criteria [3,8,13] where in the SCP subscale scores in each section of the scale were ≥ 0 because patients with mild SCP (SCP < 3) showed an early resolved PS [4]. The degree of PS was evaluated daily during physical therapy. Using the subtraction and statistical lesion analysis methods, to detect the lesion site that related the delay of recovery from PS, we divided the subjects into two groups: the recovery group, as defined by an SCP score of < 1.75 within 24 days from stroke onset (SCP < 1.75 indicates no PS [9,10]), and the no recovery group, which included patients with severe or moderate PS. We set the observation period to 24 days after onset because the minimal observation period was 24 days.
Stroke impairment was assessed according to the Stroke Impairment Assessment Set (SIAS) [18]. The Barthel index (BI) was used to evaluate activities of daily living. These tests are administered to all patients with stroke during their initial physical therapy.
Image acquisition
We used diffusion-weighted imaging (DWI) (TR/TE 6000/68.4 ms, thickness 6 mm, gap 2 mm, matrix 128 × 128, NEX 2, field of view 22 × 22 cm) and T2-weighted imaging (T2WI) (TR/TE 3000/102 ms, thickness 6 mm, gap 2 mm, matrix 320 × 256, FOV 22 × 22 cm) with the mean of 2.2 ± 2.8 days after stroke onset. DWI has proven to be particularly sensitive for the detection of hyper acute infarcts and highly accurate in predicting final infarct size [19]. Another advantage was that in very acute cases, T2WI does not affect transformation. Thus, we used DWI for lesion delineation and T2WI for spatial normalization. One very early imaging was not available because one patient was transferred from another hospital. The boundaries of all lesions were delineated directly on the image for each transverse slice using MRIcro software (www.mricro.com). Lesion volume was measured by counting the lesion voxels. Both the MRI scan and lesion shape were then mapped into stereotaxic space using a normalization algorithm provided by SPM5 (http://fil.ion.ucl.ac.uk/spm/). Automated normalization techniques can fail to accurately warp scans from individuals with brain injury, as the damaged region has a different signal intensity compared with the corresponding location in the template image. To address this problem, we used the unified model as implemented by SPM5 software for calculating transformation parameters [20]. Lesion location in the recovery and no recovery groups was compared using the subtraction technique [21]. This analysis illustrates the center of overlap in patients with recovery delay from PS in direct visual contrast to those areas that do not induce the delay of recovery from PS when lesioned. The resulting subtraction image specifically highlights regions that are both frequently damaged in patients with delay of recovery from PS as well as being typically spared in patients without one. As the two patient groups differed in sample size, we used proportional values for the MRIcro subtraction analysis.
Results
In total, 1660 in patients undergoing stroke rehabilitation (mean age, 69.9 ± 13.1 years) were included; this large sample was recruited in a previous study [11]. The length of stay for patients with stroke who received rehabilitation was a median of 26 days (range, 3-394 days). According to SCP scores, PS was observed in 156 of 1660 patients. In this study, we excluded 121 patients according to a previous study to evaluate the pure recovery from PS [11]. Of the 35 patients that were not excluded, there were nine who fulfilled the new inclusion criteria. One patient with a very small left cerebellar lesion identified by MRI was included.
The clinical characteristics of the nine patients are shown in table 1. Almost all patients had severe left hemiparesis, sensory disturbance, and unilateral spatial neglect according to SIAS. Among the nine patients with acute ischemic right hemispheric stroke, three were assigned to the recovery group and six were assigned to the no recovery group. Age, gender, SIAS motor, lesion size, sensory disturbance, unilateral spatial neglect, and first SCP scores were similar between groups (Table 2). On the other hand, the recovery group was better than the no recovery group in terms of BI and SCP after 24 days.
![]()
Table 1: Demographic and clinical data of nine ischemic right-hemisphere-damaged patients with pusher syndrome.
View Table 1
![]()
Table 2: Clinical data of patients with and without the recovery from pusher syndrome.
View Table 2
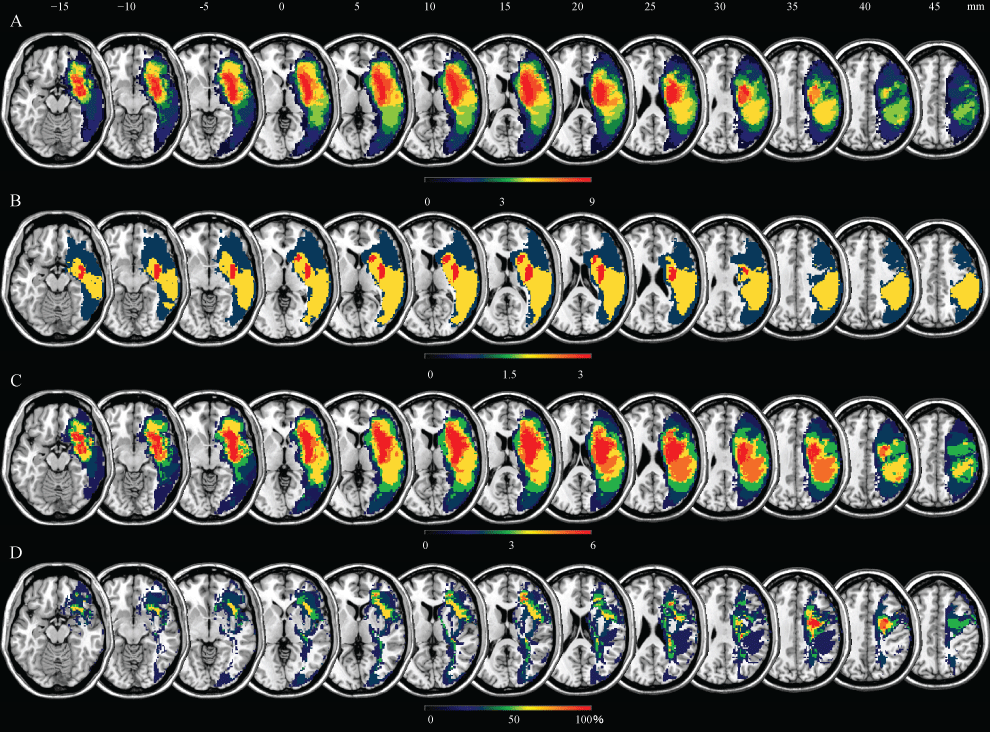
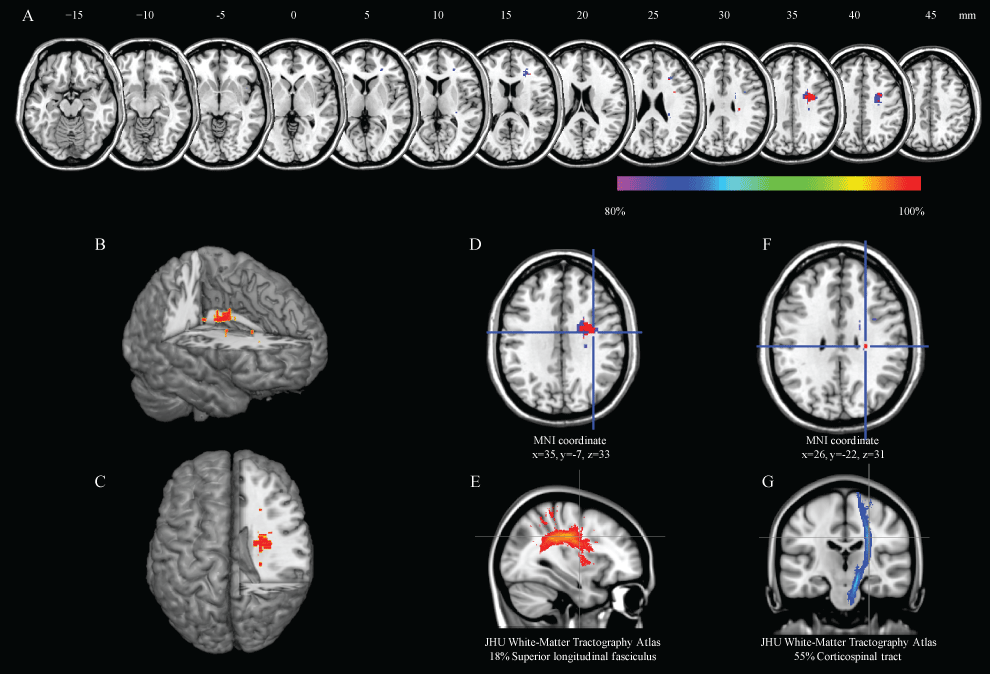
Figure 1 illustrates a conventional lesion density plot for all patients. The numbers of overlapping lesions are color coded with increasing frequencies from blue to red in all subjects. Similarly, figure 1 illustrates a lesion density plot for the recovery group, and figure 1 illustrates a lesion density plot for the no recovery group. In the subtraction images, the regions associated with delay of recovery from PS were centered on the frontal sub-cortical white matter (Figure 1). The core of overlap region is presented in figure 2. The data provide evidence for the association between the delay of recovery of PS and frontal white matter lesions. These regions corresponded to the cortico-spinal tract and superior longitudinal fasciclus. The JHU White-Matter Tractography Atlas (FSL:http://www.fmrib.ox.ac.uk/fsl/) was used as a reference for anatomical localization.
.
Figure 1: Lesion frequency distribution map in patients with pusher syndrome.
Overlay lesion plots of the nine right-hemisphere-damaged patients with pusher syndrome; A) The number of overlapping lesions are illustrated by different colors coding increasing frequencies from dark blue (n = 1) to red (n = max. number of subjects). Overlay lesion plots of the recovery group, comprising three right-hemisphere-damaged patients with recovery from pusher syndrome; B) and no recovery group, comprising six right-hemisphere-damaged patients without recovery from pusher syndrome; C) The number of overlapping lesions is illustrated by different colors coding increasing frequencies from dark blue (n = minimum) to red (n = max. number of subjects). Overlay lesion plots of the subtracted superimposed lesion of the right-hemisphere-damaged patients with recovery (recovery group) and without recovery (no recovery group) from pusher syndrome; D) The percentage of overlapping lesions of the recovery group after subtraction of the no recovery group is illustrated by different colors coding with increasing frequencies from dark blue (difference = 1%-20%) to red (difference = 81%-100%).
View Figure 1
.
Figure 2: Results of the region that related delay of pusher syndrome.
Over 80% overlap region was found the A" anatomical localization of right frontal white matter lesion by using the subtraction method, B, C) Right white matter lesions were shown with a three-dimensional rendering; D, E) These regions corresponded with the superior longitudinal fasciculus; F, G) and cortico-spinal tract. The coordinates are given in MNI (Montreal Neurological Institute) stereotaxic coordinates.
View Figure 2
Discussion
Our results show that frontal white matter lesions are consistent with right superior longitudinal fasciculus. Thus, PS induces severe failure in body posture maintenance. If PS is related to disturbed body schema, this may explain why lesions of this pathway are associated with the delay in recovery from PS.
This study revealed the association between delay in recovery from PS and right frontal white matter lesions. In the majority of stroke patients, PS resolves within several weeks [4]. One study reported, PS behavior resolved in 79% of affected patients within 3 months of acute stroke [4], and patients in another study had almost full recovery 6months after stroke onset [3]. However, the duration of the behavior widely differed among patients with PS [4,12]. Independent of the variable PS duration, the occurrence of PS per se had a significant effect on rehabilitation outcome [12]. Patients with PS are only half as efficient and effective in their rehabilitation outcome as the subgroup of patients without PS [12]. Patients with PS generally have worse outcome over a longer period of time [12]. Thus, it is important to explore the factors associated with delays in patient recovery. Babyar, et al. reported that the number of stroke impairments (motor, proprioceptive, and hemianopic or visual spatial deficit) was crucial for recovery from PS [22]. However, in our study, the severity of hemiparesis, sensory disturbance, and unilateral spatial neglect seems similar between the recovery and no recovery groups. Conversely, the recovery group was better than the no recovery group in terms of BI. PS is a postural disorder, and BI is commonly associated with the basic function of postural balance. When PS remains unresolved, it leads to lower BI. Between-group differences in SCP appear to be small but may lead to differences in the BI. The involvement of these lesions seems to be related to the delay of recovery from PS as there were no differences in factors such as hemiparesis and unilateral spatial neglect, which could be related to the delay, between the recovery and no recovery groups. Our results indicate that when patients with PS have right frontal white matter lesions, plans for a longer rehabilitation should be considered compared with patients with other lesions.
Previous studies have revealed that the occurrence of PS is associated with specific lesion sites; particularly, the posterior thalamus [13], posterior insula, and subcortical region on post-central gyrus [14]. However, it is unclear which lesion site is associated with the delay in recovery from PS.
Our results are consistent with previous reports because our study was retrospectively performed only in patients with PS. Therefore, all patients had post-insula and/or post-central gyrus subcortical lesions. A previous study reported that patients with severe PS showed severe hemiparesis and had long-term residual severe paresis [4]. Our results indicated that frontal white matter lesions were related to delays in recovery from PS; these regions involved the premotor cortex via the cortico-spinal tract. PS is highly associated with motor deficits, and other studies [23,24] have revealed that white matter lesions under the premotor cortex are conclusive evidence of less motor function recovery. Thus, our results and these previous reports are in agreement, and severe hemiparesis might be related to a delay in recovery from PS.
Other areas that are considered responsible for abnormality are of the body schema. Recent neuro imaging techniques have revealed neuronal substrates for human body schema [25]. A dynamic limb position model appears to be computed in the central motor network (represented by the primary motor cortex). Here proprioceptive (kinesthetic) signals from muscle spindles are transformed into motor commands, which may underlie somatic perception of limb movement and facilitate its efficient motor control. Somatic signals originating from different body parts are integrated in the course of hierarchical somato sensory processing, and activity in higher-order somato sensory parietal cortices is capable of representing a postural model of the entire body. Of course, posture and activity are constructed based on the body schema. The right parietal lobe is involved in the body schema; the right fronto-parietal regions connected by the most inferior branch of superior longitudinal fasciculus fibers seem to have the functions of monitoring bodily states and updating body schema [26].
Our study does have some limitations. As we tried to exclude certain factors that may affect recovery from PS, we could not amass a large number of subjects. Therefore, research on PS with larger sample sizes is required in future studies. Furthermore, in this study, we used clinical imaging that had inadequate resolution for clinical diagnosis and observation. Thus, it may be more desirable to conduct a voxel-based lesion analysis using more accurate imaging. Because this study was undertaken in acute care hospitals, we could not provide a sufficiently long observational period. It may be desirable to conduct a cooperative study with rehabilitation hospitals.
Conclusions
Previous studies revealed that patients with PS require longer rehabilitation to reach outcome goals. Our results indicate when patients with PS have right frontal white matter lesions, plans for longer rehabilitation should be considered for these patients.
Conflict of Interest
Dr. Abe received honoraria for oral presentations from gene Co., Ltd., Answer plus Co., Ltd., SESSION Co., Ltd., Epoch Co., Ltd. and Japanese Physical Therapy Association.
Acknowledgments
This work was partially supported by JSPS KAKENHI Grant Number 26120007. This study was funded, in part, by the Japanese Physical Therapy Association. The authors would also like to thank Tomohiro Chiba and the rest of the imaging staff for their skilled MRI acquisition. We also thank Enago for the English language review.
References
-
PM Davies (1985) Steps to follow: A guide to the treatment of adult hemiplegia, Springer-Verlag, New York.
-
Pedersen PM, Wandel A, Jorgensen HS, Nakayama H, Raaschou HO, et al. (1996) Ipsilateral pushing in stroke: incidence, relation to neuropsychological symptoms, and impact on rehabilitation. The Copenhagen Stroke Study. Arch Phys Med Rehabil 77: 25-28.
-
Karnath HO, Johannsen L, Broetz D, Ferber S, Dichgans J (2002) Prognosis of contraversive pushing. J Neurol 249: 1250-1253.
-
Danells CJ, Black SE, Gladstone DJ, McIlroy WE (2004) Poststroke "pushing": natural history and relationship to motor and functional recovery. Stroke 35: 2873-2878.
-
Babyar SR, White H, Shafi N, Reding M (2008) Outcomes with stroke and lateropulsion: a case-matched controlled study. Neurorehabil Neural Repair 22: 415-423.
-
S Premoselli, L Cesana, C Cerri (2001) Pusher syndrome in stroke; clinical, neuropsychological and neurophysiological investigation. Eur Med Phys 37: 143-151.
-
RW Bohannon, AC Cook, PA Larkin, WE Dubuc, MB Smith, et al. (1986) The listing phenomenon of hemiplegic patients. Neurology Report 10: 43-44.
-
Karnath HO, Ferber S, Dichgans J (2000) The origin of contraversive pushing: evidence for a second graviceptive system in humans. Neurology 55: 1298-1304.
-
Baccini M, Paci M, Rinaldi LA (2006) The scale for contraversive pushing: A reliability and validity study. Neurorehabil Neural Repair 20: 468-472.
-
Baccini M, Paci M, Nannetti L, Biricolti C, Rinaldi LA (2008) Scale for contraversive pushing: cutoff scores for diagnosing "pusher behavior" and construct validity. Phys Ther 88: 947-955.
-
Abe H, Kondo T, Oouchida Y, Suzukamo Y, Fujiwara S, et al. (2012) Prevalence and length of recovery of pusher syndrome based on cerebral hemispheric lesion side in patients with acute stroke. Stroke 43: 1654-1656.
-
Krewer C, Luther M, Muller F, Koenig E (2013) Time course and influence of pusher behavior on outcome in a rehabilitation setting: a prospective cohort study. Top Stroke Rehabil 20: 331-339.
-
Karnath HO, Johannsen L, Broetz D, Kuker W (2005) Posterior thalamic hemorrhage induces "pusher syndrome". Neurology 64: 1014-1019.
-
Johannsen L, Broetz D, Naegele T, Karnath HO (2006) "Pusher syndrome" following cortical lesions that spare the thalamus. J Neurol 253: 455-463.
-
Karnath HO, Suchan J, Johannsen L (2008) Pusher syndrome after ACA territory infarction. Eur J Neurol 15: e84-e85.
-
LF Ticini, U Klose, T Nagele, HO Karnath (2009) Perfusion imaging in Pusher syndrome to investigate the neural substrates involved in controlling upright body position. PLoS One.
-
Baier B, Dieterich M (2012) Pusher syndrome in patients with cerebellar infarctions? J Neurol 259: 1468-1469.
-
N Chino, S Sonoda, K Domen, E Saitoh, A Kimura (1994) Stroke Impairment Assessment Set (SIAS)-a new evaluation instrument for stroke patients. Jpn J Rehabil Med 31: 119-125.
-
Schaefer PW, Hunter GJ, He J, Hamberg LM, Sorensen AG, et al. (2002) Predicting cerebral ischemic infarct volume with diffusion and perfusion MR imaging. AJNR AJNR Am J Neuroradiol 23: 1785-1794.
-
Jenny Crinion, John Ashburner, Alex Leff, Matthew Brett, Cathy Price, et al. (2007) Spatial normalization of lesioned brains: performance evaluation and impact on fMRI analyses. Neuroimage 37: 866-875.
-
Rorden C, Karnath HO (2004) Using human brain lesions to infer function: a relic from a past era in the fMRI age? Nat Rev Neurosci 5: 813-819.
-
SR Babyar, MG Peterson, Reding M (2015) Time to recovery from lateropulsion dependent on key stroke deficits: a retrospective analysis. Neurorehabil Neural Repair 29: 207-213.
-
Riley JD, Le V, Der-Yeghiaian L, See J, Newton JM, et al. (2011) Anatomy of stroke injury predicts gains from therapy. Stroke 42: 421-426.
-
Lo R, Gitelman D, Levy R, Hulvershorn J, Parrish T (2010) Identification of critical areas for motor function recovery in chronic stroke subjects using voxel-based lesion symptom mapping. Neuroimage 49: 9-18.
-
Naito E, Nakashima T, Kito T, Aramaki Y, Okada T, et al. (2007) Human limb-specific and non-limb-specific brain representations during kinesthetic illusory movements of the upper and lower extremities. Eur J Neurosci 25: 3476-3487.
-
Naito E, Morita T (2014) Neural representation of human body schema and corporeal self-consciousness. Brain Nerve 66: 367-380.





