International Journal of Neurology and Neurotherapy
Abnormal Plasticity and Epigenesis in Epileptic Seizures of an EL Mouse
Jiro Suzuki*
Sannou Psychiatric and Psychological Institute, Tokyo, Japan
*Corresponding author:
Jiro Suzuki, Sannou Psychiatric and Psychological Institute, Daiichi Matsuura Bldg. 3rd Fl, Akasaka Minato-ku, Tokyo, 107-0052, Japan, Tel: 81-3-5561-6211, E-mail: jsuzuki@rinsen-cl.com
Int J Neurol Neurother, IJNN-3-059, (Volume 3, Issue 6), Rather Short Review; ISSN: 2378-3001
Received: November 08, 2016 | Accepted: November 28, 2016 | Published: November 30, 2016
Citation: Suzuki J (2016) Abnormal Plasticity and Epigenesis in Epileptic Seizures of an EL Mouse. Int J Neurol Neurother 3:059. 10.23937/2378-3001/3/6/1059
Copyright: © 2016 Suzuki J. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Summary
The abnormal plasticity and epigenetic phenomenon observed in the development of epileptic seizures and developmental process of an EL mouse, which is an excellent mutant model, are reviewed through the neurophysiological, biochemical and molecular aspects.
The EL mice were exposed to the natural proprioceptive sensory stimulation of tossing the animal into the air to provoke an epileptic seizure, which results from an increase in the excitability of the cortical neurons due to low activities of Glutamic acid decarboxylase (GAD) and Gamma-amino butyric acid (GABA). The seizures develop due to the abnormal plasticity and eventually occur spontaneously. At the same time the provoked seizures, in turn, may induce DNA fragmentation or Immediately Early Gene (IEG) expression and make some changes of neurotrophic factors and protein synthesis. These proteins or other substances may constitute another structure vulnerable to provocative stimuli to the seizure.
Keywords
EL mouse seizure, Natural proprioceptive sensory stimulation, Parietal cortex and hippocampus, GAD-GABA activity, Abnormal plasticity, DNA rearrangement
Introduction
Epilepsy is known to be a highly complicated disease or syndrome [1]. The EL mouse is an established animal model of epilepsy [2], and several studies have investigated the epileptogenesis of this disease using the EL mouse model [3-5].
Long-term potentiation (LTP), which was discovered by Bliss and Lomo [6] is considered to be the cellular correlate of learning and memory [6,7], and LTP-induced plasticity is believed to confer beneficial effects to an organism. In contrast, epileptic seizures in the EL mouse are provoked by natural proprioceptive sensory stimulation, including repeatedly tossing-the animal into the air [8,9] or by performing rotating (see saw) movements [10,11], but are not induced by loud sounds or vestibular stimulation. In addition, the EL mouse seizure still occurred even after the labyrinths were destroyed, at that time the animal could not maintain its posture. Therefore, the seizure-provoking mechanism in the El mouse occurs through the proprioceptive nervous system from abrupt accelerating movements [8].
We called the process of developing unfavorable results in an organism as abnormal plasticity in contrast to the neural plasticity [9]. The abnormal plasticity could be considered as one of pathogeneses of the nervous diseases [4]. Also kindling by repetition of electric stimulation to the brain or frequent administration of central stimulants, e.g. cocaine are associated with progressively increasing seizures or pathological behaviors, which are artificial, unfavorable and distinctly noxious effects on an organism [12,13].
The seizure susceptibility of EL mouse is seemingly autosomal dominant in genetics [14,15]. However this susceptibility is successively increased by naturally sensory stimulation to the animals [8]. This abnormal plastic process represented by symptoms or seizures and electrophysiological aspects was demonstrated in some previous reports [4,5,8,9].
In the present paper some biochemical and molecular aspects of the abnormal plastic process, which can be considered as one of the pathogeneses of epilepsy, and especially an epigenetic aspect of EL mouse seizure are reviewed.
Subjects and Methods
The details of the experimental animals and various experimental procedures were described precisely in our previous papers [3-5,8,9,13,16-23] but are briefly summarized here.
Experimental animals
One hundred animals each of the EL*/Suz line and DDY line were used for the investigation. Mice of both genders were used, and they weighed 26-30 g and were 20 to 50 weeks old. No sexual difference in the occurrence of seizure was found.
*This mutant mouse strain was named "ep" when it was first discovered, was later named "El", and was then renamed "EL" at the International Symposium in Tokyo in 1992. The provider of the animals should manifest this strain to be the EL strain, which deprived from Suzuki (e.g. Leussis, Heinrichs, 2007) [11].
All animals of the EL/Suz strain were inbred to the F128 generation, and those of the DDY strain were inbred to the F66 generation. The animals were carefully reared at our institute and were treated with the routine provoking stimulation of being tossed up in the air once a week since 4 to 5 weeks of age [9]. At first, no animal seized, but after the provoking stimulation had been repeated several times, all animals easily seized and, eventually, they seized almost spontaneously. Thus, the threshold for seizing was lowered by repetition of the provoking stimulation, as described in the literature [8], ensuring that they were extremely highly prone to seizure. The history of the development of seizure is described precisely in the previous papers [5,8,9,21]. No animal of the DDY strain seized, even after being treated with similar repeated provoking stimulation.
Methods
1) Chronic physiological experiment.
The animals were anesthetized (pentobarbital, 50 mg/kg body weight) and fixed in a stereotaxic apparatus (Narishige, Tokyo, modified by the author) during electrode implantation. The electric activities of several portions of the brain were chronically recorded through thin stainless steel microelectrodes, which were stereotaxically implanted according to the Sid man's atlas [24], while the mice were freely moving.
2) In the acute physiological experiment, the firing of unitary neurons was recorded via stereotaxically inserted microelectrodes by a bioelectric amplifier (Nihon Kohden) and was analyzed using Spike 2 software (Cambridge). After recording the data, the positions of the tips of the microelectrodes were verified by applying an electric current.
3) To observing one of the metabolic aspects, the [14C] 2-deoxyglucose technique by Sokoloff, et al. [25] was used [20,22].
4) Various kinds of neurochemical aspects were reported so far in our investigation, in which GABA and GAD activities studied gave us particularly important data to find the way to epileptogenesis. In our investigation with using micro neurochemical methods, freeze-dried micro brain region samples were used for measuring GABA concentration and GAD activities in epileptogenesis. The ultra-micro enzymatic chemistry of NADPH cycling with fluorometric assay was used to measure activities of those substances in the parietal cortex and hippocampus [17].
5) Cytokines, such as interleukin-1 alpha (IL-1a), IL-1b, IL-6, IL-1 receptor (IL-1r), IL-1receptor antagonist (IL-ra) and tumor necrosis factor alpha (INF-a) were examined by Western blotting in the parietal cortex and hippocampus of EL mice [18].
6) For investigating molecular aspects of epileptogenesis, IEG expression (c-fos and zif) was analyzed by in situ hybridization by using 35S [19]. DNA fragmentation was detected by in situ terminal transferase-mediated dUTP Nick labelling with the aid of alkaliphosphatase and peroxidase (TUNEL method).
Results
1) Abnormal plasticity as a pathogenesis of epileptic seizure of an EL mouse [9].
In the EL mouse seizure provoked by tossing-up stimulation, at first few EL mice of 4 to 5 weeks of age seized but after about 20 weeks all EL mice easily seized and eventually they seized almost spontaneously. The seizure threshold was lowered by repetition of the stimulation. But after a long period of no stimulation, the thresholds were raised, or extinction of seizures was observed in mice even with fully developed seizures. Reinforcement of stimulation was necessary for the seizures of the EL mice to occur. During recovery of the occurrence of the seizures, their incidence increased sooner than in the previous developmental period [5,9].
2) The abnormal plasticity is also represented by changing and growing electroencephalographic discharges [2,5,8,12]. Paroxysmal discharges occur at first at the parietal cortex followed by the hippocampus or other cerebral portions in response to the proprioceptive sensory inputs by tossing up procedure. The paroxysmal discharges induced various forms of seizures as described in the previous papers [21]. All individual seizures repeat the developmental process of seizures in order [5].
No other way of abrupt accelaration movement than tossing-up or sea saw movement can provoke a seizure at the steady level in EL. These provoking stimuli are transmitted via the proprioceptive sensory pathway to the cortex (not by vestibular input [8,9,26].
3) In relation to these behavioral and neurophysiological phenomena, various neurochemical aspects were observed in the cerebral cortex, particularly the parietal and hippocampal neurons. (The focus complex by our first expression [4] in an EL mouse brain. In these brain areas epileptic discharges initially start at the parietal cortex and are boosted to manifest clinical symptoms).
The paroxysmal neuronal firings at the seizures increased the metabolic activities expressed by glucose intake into the cerebral cortical and hippocampal neurons, which could be observed by the [14C] 2-deoxyglucose technique [20,22,25].
4) In the bases of paroxysmal neuronal discharges there could be found decreased activities of the inhibitory mechanism. Especially GABA concentrations and GAD activities show important results to find the way to epileptogenesis. Decreasing concentrations of GABA at the parietal cortex and hippocampus were observed in parallel with changes of firing activities in response to the proprioceptive sensory inputs and increased paroxysmal firing activities of neurons [3,17,23,27].
The abnormal plasticity is also represented by decreasing process of GABA-related inhibitory activity [17], in other words increasing of the excitability of the cortical neurons and hippocampus. It means the neuronal activities themselves in the laminar structure of those areas might be changed by the influences of the seizures [23].
5) Some important observations in relation to development of EL mice and consequences caused by seizures themselves which may relate to the epileptogenesis and moreover to epigenesis [3,8,16-19,27] are summarized as following.
In EL mice, which had experienced seizures and lower thresholds, DNA fragmentation without cell loss was detected in the parietal cortex and hippocampal CA1 [8,27]. EL mice also showed lower activities of Cu, Zn-super oxide dismutase (Cu, Zn-SOD) than those which the control DDY mice did [18]. These findings suggest that abnormalities in the region-specific Cu, Zn SOD isozyme activity might produce spatially specific free radicals, leading to DNA fragmentation. This might contribute to epileptogenesis and the possibility of change in genome DNA.
Nitric oxide (NO) system, which is associated with synaptic plasticity, was studied referring to development and seizures of EL mouse [18]. However, one of the isozymes, e-NOS showed relatively low concentration. This fact should need more precise studies in relation to abnormal plasticity, referring to the work of Kandel's group [28]. Significant increases in the level of cytokines (IL-1 alpha, IL-1 beta, IL-receptor, IL-1 receptor antagonist, IL-6 and Tumor necrotizing factor (TNF) were observed in the parietal cortex and hippocampus at any stages during development of EL mice [3,18]. Also cytokines increased predominantly before experiencing frequent seizures. In the parietal cortex cytokines were most highly expressed 2 hours after seizures. Cytokines were kept up-regulated until next seizures in the hippocampus whereas they were transiently up-regulated immediately after seizures in the parietal cortex.
Various neurotrophic factors were over-expressed during the growth of EL mice. Brain depived neurotrophin factor (BDNF) showed a biphasic over-expression during 8-12 weeks postnatal and also around 20 weeks of age which covers the late period of epileptogenesis [3,19]. NT-3 (a member of the neurotrophin family) showed a peak expression from 8 to 12 weeks old, corresponding to the early period of epileptogenesis. Although seizure activity itself induces expression of neurotrophic factor mRNAs and proteins, the level of NT-3 and BDNF in the hippocampus of EL mice shows a significant increase in the earlier developmental periods, before the animals exhibit seizures frequently.
6) A neurotransmitter-induced long-term plasticity requires RNA and protein synthesis during a critical time period immediately following stimulation. In the abnormal plasticity process as the EL mouse seizure frequent expression of immediate early genes (IEGs; zif and c-fos) were observed. These expressions were investigated precisely in relation to the seizure history, seizure threshold and development of EL mice [19,27]. These expressions can be considered to relate to epileptogenesis of the EL mouse.
Further Discussions Based on the Results Obtained
Here the abnormal plasticity shown by the epileptic seizure of an EL mouse provoked by the proprioceptive sensory stimulation with the natural method as stated above could be considered as a new one of pathogenetic processes of nervous system, as abnormal immunological reactions, e.g. Welsh, et al. [29]. Moreover, this process may exert unfavorable effects on the evolution of an animal without any direct manipulation of DNA. One can consider this process as one of epigenetic phenomena [4,5,9,19,23] and may remind of Thomson' review "Epigenesis and Plasticity in the nervous system" published a quarter century ago [30].
Natural proprioceptive sensory stimulation of tossing-up provokes an epileptic seizure of the EL mouse which is caused by increase of excitability of the cortical neurons. These cortical neurons have a poor GAD-GABA system or other abnormal-level constituents, which may be mutant origin at first. The seizures develop in the abnormal plastic process and finally they occur spontaneously with a weak or no provoking stimulus. At the same time the provoked seizures, in turn, may cause DNA fragmentation or IEG expression and make some kind of neurotrophic factors and protein synthesis. These proteins or other substances may constitute other structures vulnerable to provocation stimuli to the seizure. These facts remind us of the old statements "a seizure may call another seizure" as Foerster said [31].
Conclusion
In summary one can say as following, the epileptogenesis of the EL mouse seizure is induced by a natural proprioceptive sensory stimulation, which results in the development of epilepsy, but in turn the seizures themselves may induce epigenetic changes. This paradigm might offer a new insight to the pathogenesis, epigenesis and evolution of epilepsy (Figure 1).
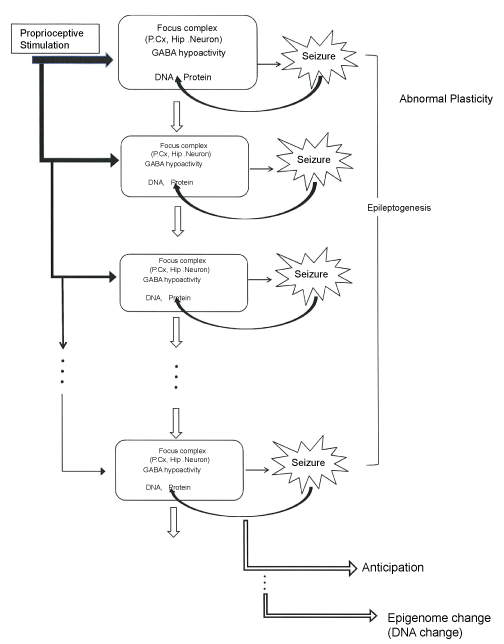
.
Figure 1: Schematic representation of abnormal plasticity, epileptogenesis, anticipation and epigenome change in the EL mouse seizure.
View Figure 1
Conflicts of Interest
This research was carried out under the authority and funding of Sannou Institute of Psychiatry and Psychology and Tokyo Institute of Psychiatry. The author has no conflicts of interest to disclose. Both institutes supplied financial support, and the author belonged to both institutes when the study was conducted and the manuscript was prepared. The author confirms having read the Journal's position on issues regarding ethical publication and affirms that this report is consistent with those guidelines.
Ethics
The author has read and abided by the statement of the ethical standards for research involving laboratory animals described by the US NIH Office of Laboratory Animal Welfare. All animal care and procedures were conducted in accordance with the IACUC (No. 21-18, 2002) of my institution, which was officially approved by the Japanese Society of Experimental Animals (an internationally approved society). An important member of the committee is a veterinarian. In addition, all possible efforts were taken to minimize animal suffering and to reduce the number of animals used in the experiment. Additionally, the author referred to the standards for the publication of mouse mutant studies written by Crusio, et al. [26] (2009) for all experiments conducted.
Acknowledgment
The author expresses sincere thanks to Dr. N Ozawa, Ms. Y Nakamoto, Dr. YL Murashima, Ms. Y Niikawa, Ms. A Nakatugu, Dr. N Ishida, Dr. A Takazawa, Dr. K Kasamo, Dr. Y Shinkawa, Dr. M Yosii, Dr. T Shinba, Ms. T Shinozaki, Dr. R Nonaka, Mr. S Nakayama, Mr. K Kato, Ms. K Matuoka, Mr. S Niibe, Mr. M Okazaki and all staff members of the Institute.
References
-
Jackson JH (1931) On the scientific and empirical investigation of epilepsies. In: Taylor J, Selected writings of John Hughlings Jackson. Hoddr and Stoughton Ltd London, 162-273.
-
Suzuki J (1976) The paroxysmal discharges of the EEG in El mouse. Experientia 32: 336-338.
-
Murashima YL, Suzuki J Yoshii M (2005) Ictogenesis and epileptogenesis in epileptic mutant EL mice. In: Benjamin SM, Focus on Epilepsy Research. Nova Science, New York, 139-198.
-
Suzuki J (2013) Neuronal mechanism of epileptogenesis in EL mouse. Proc Jpn Acad Ser Biol Sci 89: 270-280.
-
Suzuki J (2016) Development of a seizure and epileptogenesis in EL mouse. British J Medicine Medcal Res 13: 1-5.
-
Bliss TV, P Lomo T (1970) Plasticity in a monosynaptic cortical pathway. J Physiol 207: 21.
-
Lloyd DPC (1949) Post-tetanic potentiation of response in monosynaptic reflex pathways of the spinal cord. J gen Physiol 33: 147-170.
-
Suzuki J, Murashima YL, Kasamo K, Ishida N, Takazawa A (1993) Etiology of psychiatric and neurological disorders and abnormal plasticity. In: Neurotransmitters in neuronal plasticity and psychiatric disorders. Toru M Excerpta Medica International Congress Series 1061, 64-80.
-
Suzuki J, Nakamoto Y (1982) Abnormal plastic phenomena of sensory-precipitated epilepsy in the mutant El mouse. Experimental neurology 75: 440-452.
-
FuetaY, Mita T, Matsuoka S (1983) Experimental animal epilepsy: a new device for the induction of epileptic seizures in the murine, EL mouse. JUOEH 5: 359-364.
-
Leussis MP, Heinrichs SC (2007) Temporal ontogeny of circuit activation prior to the onset of seizure susceptibility in EL/Suz mice. Neuroscience 145: 33-41.
-
Goddard GV (1967) Development of epileptic seizures through brain stimulation at low density. Nature 214: 1020-1021.
-
Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ (1987) Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 237: 1219-1223.
-
Imaizumi K, Ito S, Kutukake G, Takizawa T, Fujiwara K, et al. (1959) Epilepsy like anomaly of Mice. Exp Animal 8: 6-10.
-
Rise ML, Franklel WN, Coffin JM, Seyfried TN (1991) Genes for epilepsy mapped in the mouse. Science 253: 669-673.
-
Ishida N, Kasamo K, NakamotoY, Suzuki J (1987) The roles of parietal cortex and hippocampus in the seizure of the El mouse. J Psychiat Neurol 41: 498-499.
-
Murashima YL, Kasamo K, Suzuki J (1992) Developmental abnormalities of GABA ergic system are in the formation of Epileptogenesis in the EL mouse. Neurosciences 18: 63-73.
-
Murashima YL, Suzuki J, Yoshii M (2008) Role of cytokines during epileptogenesis and in the the transition from the interictal to the ictal state in the epileptic mutant EL mouse. Gene Regulation and System Biology 27: 267-274.
-
Murashima YL, Suzuki J, Yoshii M (2009) Specific gene expression before and after seizure in eopleptic EL mice, In: Schwartzkroin P, Encyclopedia of Basic Epilepsy Research. Elsevier, New York, 253-259.
-
Nakamoto Y, Nakayama K, Suzuki J (1990) Cerebral uptake of 14Cdeoxyglucose during the entire seizure and the recovery period in an El mouse. Epilepsy Res 5: 43-48.
-
Suzuki J, Nakamoto Y (1977) Seizure patterns and electroencephalograms of the El mouse. Electroencephalogr Cli Neurophysiol 43: 299-311.
-
Suzuki J, Nakamoto Y, Shinkawa Y (1983) Local cerebral glucose utilization in epileptic seizure of the mutant El mouse. Brain Res 266: 359-363.
-
Suzuki J, Ozawa N, Murashima YL, Shinba T, Yoshii M (2012) Neuronal activity in the parietal cortex of EL and DDY mice. Brain Res 1460: 63-72.
-
Sidman RL, Angevine Jr, JB Taber, Pierce E (1971) Atlas of the Mouse Brain and spinal cord. Harvard Univ Cambridge Mass.
-
Sokoloff L, Reivich M, kennedy C, Des Rositers MH, Patlak CS, et al. (1977) The [14C] deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 28: 897-916.
-
Crusio WE, Goldowitz D, HolmesA, Wolfer D (2009) Standards for the publication of mouse mutant studies. Genes Brain Behav 8: 1-4.
-
Murashima YL, KasamoK, Suzuki J (1996) Development and seizure-related regional differences in immediate early gene expression and GABAergic abnormalities in the brain of EL mice. Epilepsy Research 26: 3-14.
-
Barzilai A, Kennedy TE, Sweatt JD, Kandel ER (1989) 5-HT modulates protein synthesis and the expression of specific proteins during long-term facilitation of specific proteins during long-term facilitation in Aplysia sensory neurons. Neuron 2: 1577-1586.
-
Welsh FA, Foyer DJ, Harris VA (1992) J Cerebral Blood Flow Metabolism 12: 204-212.
-
Todorova MT, Mantis JG, Le M, Kim CY, Seyfried TN (2006) Genetic and environ-mental interactions determine seizure susceptibility in epileptic EL mice. Genes Brain Behav 5: 518-527.
-
Foerster O (1926) Die Pathogenese des epileptischen Krampfanfalles, Klinischer Teil, Dtsch Z Nervenheilk, 94.





