Iron oxide nanoparticle is the most promising nanoparticles (NPs) capable in Drug Delivery and targeting. Iron oxide nanoparticles were synthesized by green synthesis. Galactomannan, when attached to the surface of the nanoparticles, increases the biocompatibility of the nanoparticles. Folic acid (FA) is used as the ligand to target folate receptors, which are found abundant in cancer cells. FeNPs-GM-FA could target cancer cells when used as drug carriers. The synthesized iron oxide nanoparticles using Mimosa pudica root extract was synthesized for targeted delivery of the anticancer drug, Capecitabine, by grafting folic acid (FA) onto the iron oxide nanoparticles coated with galactomannan (GM), a polysaccharide present in fenugreek gum. The cytotoxicity profile of the nanoparticles on human epithelial type 2 (HEp-2) cells as measured by standard 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay showed that the particles were nontoxic and may be useful for various in vivo and in vitro biomedical applications. The surface modification by galactomannan and folic acid grafting was confirmed by UV-visible spectroscopy and fourier transforms infrared (FTIR) spectroscopy. The in vitro release profile of capecitabine from FeNPs-GM-FA was characterized by an initial fast release followed by a sustained release phase. The histological investigation evidences the formation of improved liver cell architecture indicating the therapeutic nature of functionalized iron nanoparticles with Capecitabine, confirming a potential option for drug delivery and targeting tumor tissues.
Iron oxide nanoparticles, Galactomannan, Folic acid, Drug targeting, Capecitabine
Targeted drug delivery is the process of delivering drug specifically to a cell, tissue and organ where a pharmacological activity is required. It expands the therapeutic window of the drug by delivering the drug to the therapeutic site and by reducing delivery to the unwanted site. This decreases the minimum effective dosage of the drug compared to the usual route and thus reduces the toxic side effects of the drug [1].
Magnetic nanoparticles (MNPs) possess unique properties that are the ability to function at the cellular and molecular level of biological interactions making them an attractive platform as contrast agents for magnetic resonance imaging (MRI) and as carriers for drug delivery [2]. Nanoparticles are used for drug targeting because the diameter of the smallest blood capillary is approximately 5 μm. It can be penetrable into the smallest blood capillaries. The nanoparticles (NPs) used should be specific, efficient and rapidly internalized into specific target cells. Coating the nanoparticles with biocompatible polymers and targeting agents can improve the dispersive and biocompatibility of nanoparticles [3].
The therapeutic agents are attached or encapsulated within a magnetic nanoparticle. These particles may consist of porous polymers that contain magnetic nanoparticles precipitated within the pores or may have magnetic cores with a polymer or metal coating which can be functionalized. By functionalizing with the polymer or metal coating, it is possible to attach a drug to cure a disease. Once attached, the particle/drug complex is injected into the bloodstream. Magnetic fields generally from high-field, high gradient, rare earth magnets are focused over the target site and the forces on the particles as they enter the field allow them to be captured and extravagated at the target [4].
The green synthesis of nanoparticles is a novel approach in nanotechnology. Plants have been reportedly used for synthesis of metal nanoparticles like gold, silver, iron, copper and alloy. Iron oxide nanoparticles (FeNPs) is a unique micro configuration and super-para magnetism. Super-para-magnetic iron oxide nanoparticles with tailored surface chemistry have been widely used in numerous applications such as magnetic resonance imaging (MRI) contrast enhancement, tissue repair, immunoassay, detoxification of biological fluids, hyperthermia, drug delivery, cell separation, etc. All these biomedical and bioengineering applications require that nanoparticles have high magnetization values and size smaller than 100 nm with overall narrow particle size distribution, so that the particles have uniform physical and chemical properties. In addition, these applications need special surface coating of the magnetic particles, which must be not only nontoxic and biocompatible but also allow a targetable delivery with particle localization in a specific area [5].
Polymers are coated on the nanoparticle surface to disperse it and make them more biocompatible. Polymer prevents the nanoparticles from agglomeration and particle surface from oxidation. Galactomannans, an important group of polysaccharide hydrocolloids, are produced in certain plants as cell wall and storage polysaccharides. Galactomannans generally consist of β, 1-4-linked linear mannose backbone, to which single galactose grafts are randomly linked by α, 1-6 glycoside bond. The structure of galactomannan is shown in Figure 1. Galactomannans from different legume seeds differ in mannose to galactose ratio, molecular weight and mode of placement of the galactose grafts. Galactomannan derived from the seeds of Trigonellafoenum-graecum (Comman name: Fenugreek) has mannose: galactose ratio of 1:1. Thus Fenugreek gum has the highest galactose (~48%) in its molecule and its linear mannan backbone has α, 1→6 linked single galactose grafts on nearly all the mannose groups of the main chain. A gum with higher percentage of galactose has good cold-water dispersability and high viscosity [6].
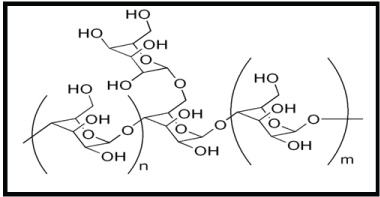 Figure 1: Structure of Galactomannan. View Figure 1
Figure 1: Structure of Galactomannan. View Figure 1
Folate receptor (FR) is an affinity membrane folate-binding protein. It is a glycosyl phosphatidylinositol (GPI)-linked membrane glycoprotein with an apparent molecular weight of 38-40 KDa. Its expression is negligible in healthy cells and is present in large numbers in cancer cells. Highly undifferentiated metastatic cancer sites express more FR. Over expression of FR on cancer cell makes it as a potential target for a ligand. Folic acid (FA), a low molecular weight targeting agent can be coated on nanoparticles as a ligand as it can couple to the folate receptor. FA is grafted on the polymer to target cancer cells [7]. The structure of folic acid is shown in Figure 2.
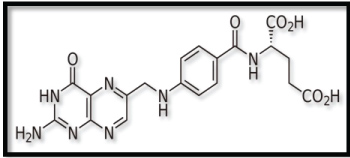 Figure 2: Structure of Folic Acid. View Figure 2
Figure 2: Structure of Folic Acid. View Figure 2
This paper discusses the green synthesis of Iron nanoparticles and its conjugations with anti-tumor drug and the in-vitro drug release response of galactomannan functionalized iron oxide nanoparticles attached to folic acid and conjugated with the anti-cancer drug capecitabine as a model drug. The FeNPs incorporated with FA, galactomannan and drug was characterized using Scanning electron microscopy (SEM), particle size analyzer and UV-Visible and FT-IR spectroscopy. The pharmacological potential of functionalized FeNPs was investigated by cell culture and histological studies.
The green synthesized FeNPs prepared in our laboratory was used in this study. All chemicals used for the synthesis of FeNPs were purchased from Sigma-Aldrich, India. Double distilled water was used throughout the experiment. All glassware was washed well and dried using hot air oven (Figure 3).
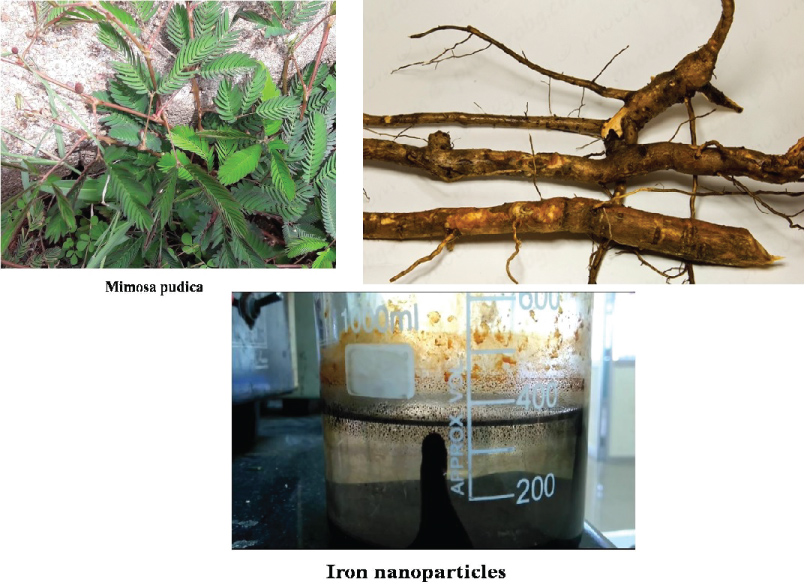 Figure 3: Iron Nanoparticles synthesized via Greener approach using Mimosa pudica.
View Figure 3
Figure 3: Iron Nanoparticles synthesized via Greener approach using Mimosa pudica.
View Figure 3
Fresh fenugreek seeds were washed thoroughly with distilled water and were dried completely. The dried seeds were crushed into powder. 5 g of powder was soaked in 50 ml of distilled water and was stirred continuously for 4 hours. It was then centrifuged at 6000 rpm for 15 mins. The supernatant, which is the fenugreek gum, was collected and stored for further process.
The green synthesized Iron nanoparticles (FeNPs) is characterized by UV-Visible spectroscopy. The reduction of pure Iron nanoparticles was primarily monitored by measuring the UV-visible spectrum of the reaction medium after diluting the solution with the range between 100-900 nm in UV-visible spectrophotometer-vis absorption spectrum measures the wavelength of the light that the nanoparticles absorb. UV-spectrum is shown in the Figure 4. It shows a broad peak of Iron nanoparticles to detect the presence of Iron nanoparticles in the solution. Green synthesis of Iron nanoparticle was achieved using the plant as the color change was observed after the addition of ferrous sulphate solution due to the excitation of Surface Plasmon Resonance (SPR). In UV-visible spectrometer, the absorbance spectrum was measured.
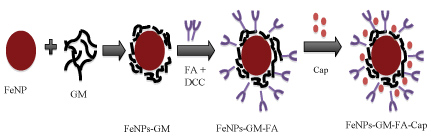 Figure 4: Schematic representation of FeNPs-GM -FA-Cap. View Figure 4
Figure 4: Schematic representation of FeNPs-GM -FA-Cap. View Figure 4
The surface appearance and morphology of the green synthesized iron particles are evaluated by Scanning electron microscopy. It was performed by SEM built up by utilizing Supra Zeiss with 1 nm determination at 30 kV with 20 mm Oxford EDS finder.
To synthesize Polymer-functionalized iron oxide nanoparticles, the iron oxide nanoparticles were mixed with the fenugreek gum, which was extracted earlier in the mass ratio of 1:1 and stirred overnight. The polymer-coated nanoparticles were then centrifuged at 6000 rpm for 15 mins. The polymer coated iron oxide nanoparticles were collected, washed with distilled water and dried.
Folic acid is generally difficult to conjugate to the surface of the polymer because of the weak chemical reactivity. The carboxylic group of folic acid was first activated with dicyclohexylcarbodiimide (DCC). DCC activates the folic acid and iso-urea is formed. 10 mg of Folic acid was dissolved in 10 ml of dimethyl sulfoxide (DMSO) solution. DCC was added to the solution corresponding to a ratio of folic acid to DCC 1:1. Galactomannan-functionalized iron oxide nanoparticles were added in the folic acid solution in the mass ratio of 1:1. It could soak for 1 hour. The nanoparticles were then separated by centrifugation, washed with distilled water and dried (5.7).
The anticancer drug Capecitabine was dissolved in DMSO. Folic acid modified Polymer functionalized nanoparticles was added to drug solution (drug concentration = 1 mg/ml) in a mass ratio of 1:1 and stirred at room temperature for 4 hours. The suspension was then centrifuged (6000 rpm, 15 min) and the precipitate were separated and dried. The schematic representation of FeNPs-GM-FA-Cap is given in Figure 4.
For in-vitro drug release study, Dialysis Membrane-110, LA395 (Membrane specifications: flat width - 31.13 mm, Av. diameter - 21.5 mm, capacity approx - 3.63 ml/cm) was used. 100 mg of FeNPs-GM-FA was taken along with 10 mg of Capecitabine. Then the sample (FeNPs-GM-FA-Cap) was packed in the dialysis membrane and immersed in phosphate buffer saline (PBS) solution (150 ml) with mild stirring at 37 °C throughout the process. 2 ml samples were withdrawn by a pipette at 30, 60, 80, 120, 150, 180, 240, 300, 360, 1440 and 4320 minutes and replaced immediately with 2 ml of fresh PBS medium, which was accounted for when calculating the amount released. Capecitabine concentration in the collected samples was measured using UV-Vis spectrophotometer at a wavelength of 307 nm. The percentage of drug released is given by,
Where, A is the amount of initial capecitabine that was incorporated into the folic acid modified GM-functionalized iron oxide nanoparticles and B is the amount of drug released at a time, t. The data are collected and statistically analyzed from the triplicate if the experiments. The standard deviation of the analysis is expressed in error bars in the graphs.
The cytotoxicity of the synthesized iron oxide nanoparticles was evaluated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. 100 μl of HEp2 cells were seeded in 96-well plates (5 × 104 cells/well) and they were kept for overnight incubation at CO2 incubator. The condition of CO2 incubator was maintained at a temperature of 37 °C and cell were humidified 5% CO2 in the incubator for 24 h.
The iron oxide nanoparticles were then added to the cells at defined concentrations (2, 6, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100 μg/ml) and incubated for 24 hours. After incubation, the media was discarded and 50 μl of MTT reagent (5 mg/ml) was then added per well and the plate was incubated for 4 hours. After incubation, the media with MTT was discarded from the wells and 100 μl of dimethyl sulfoxide (DMSO) was added to solubilize the formazan crystals formed. Readings were then taken in an enzyme-linked immunosorbent assay (ELISA) reader at 570 nm. Percentage viability of cells was calculated as the ratio of mean absorbance of triplicate readings with respect to mean absorbance of control wells as shown in the equation 2.
Where, Isample is the mean absorbance of triplicate readings of sample and Icontrol is the mean absorbance of control. The error bars in the graph concludes the standard deviation of the statistical analysis of the triplicate experiments.
Histological evaluation was analyzed on a lobe of the liver tissue and a part of the specimen was fixed using 10% formalin and then embedded in paraffin wax, sectioned at 4l m thickness and were stained with hemotoxylin-eosin. Normal light microscopy was used to evaluate pathological changes of liver.
The spectra recorded from the FeNPs-GM solution, shows a shift in the onset of absorption observed in the sample and showed an absorbance peak at 224 nm, which was specific for the Iron oxide nanoparticles. This phenomenon of shift of absorption edge has been ascribed to a decrease in particle size. This results in a shift in the absorption edge to a lower wavelength region. Furthermore, nanoparticles are highly sensitive and functionally efficient because of smaller grain size and high surface to volume ratio as compared to the conventional materials in micrometer range (Figure 5).
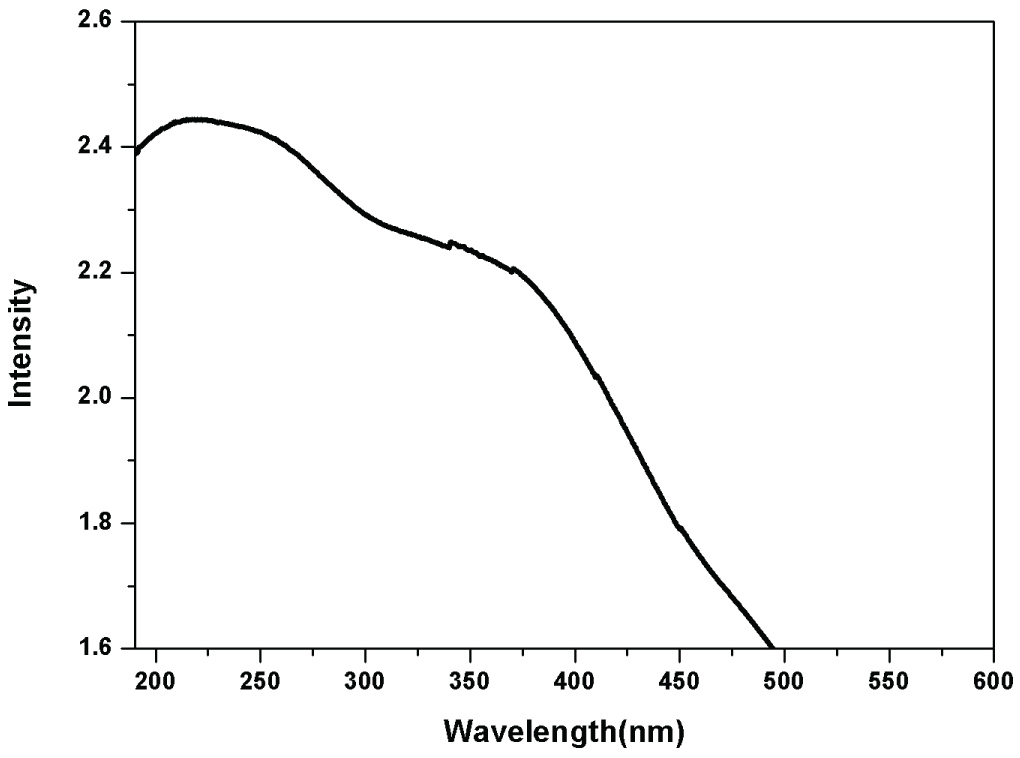 Figure 5: UV-Visible spectrum of iron nanoparticle via green synthesis.
View Figure 5
Figure 5: UV-Visible spectrum of iron nanoparticle via green synthesis.
View Figure 5
SEM analysis was used to confirm the morphology of the synthesized FeNPs-GM sample. SEM image of Galactomannan coated iron oxide nanoparticles demonstrates that these particles are smaller than 100 nm and have spherical shape (Figure 6).
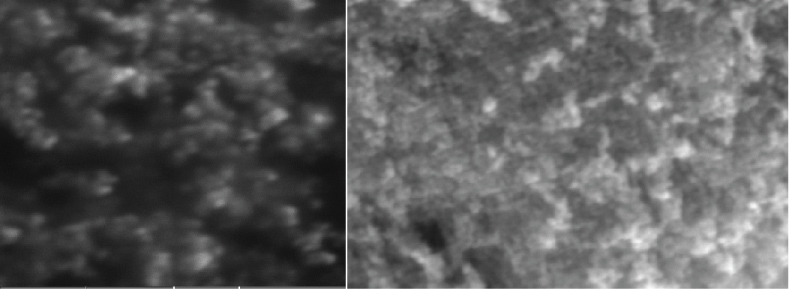 Figure 6: SEM Micrographs of Galactomannan coated FeNP via green synthesis.
View Figure 6
Figure 6: SEM Micrographs of Galactomannan coated FeNP via green synthesis.
View Figure 6
EM image shows that the synthesized iron oxide nanoparticles are well dispersed and roughly spherical in shape. The synthesized iron oxide nanoparticles are in the size range of 60-80 nm with mean size of 67 nm.
The size of FeNPs via green synthesis is found to be 167.2 ± 55.44 nm and the size Of FeNPs after surface functionalization is 202.3 ± 45.71 nm and surface activation of nanoparticles enhanced its size by forming a layer on the nanoparticles. Figure 7 shows the particle size of iron particles before and after functionalization.
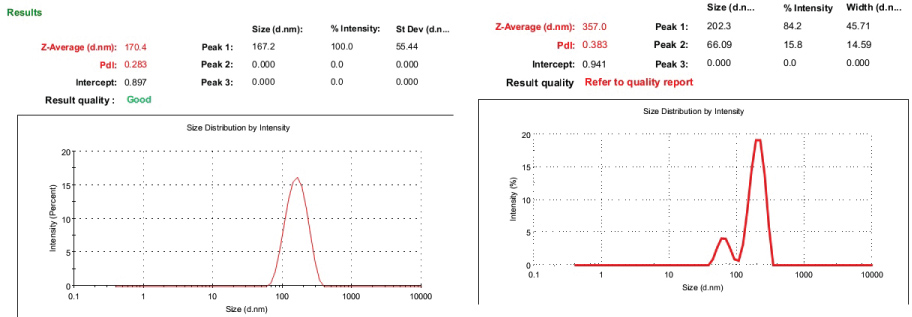 Figure 7: A) Iron nanoparticles average particle size; B) Particles average size of surface functionalized iron nanoparticles.
View Figure 7
Figure 7: A) Iron nanoparticles average particle size; B) Particles average size of surface functionalized iron nanoparticles.
View Figure 7
Figure 8 shows the FTIR spectrum of galactomannan coated iron oxide nanoparticles, folic acid modified galactomannan coated iron oxide nanoparticles and folic acid modified galactomannan-coated iron oxide nanoparticles conjugated with capecitabine. In the FTIR spectrum of FeNPs-GM, the peak at 3402 cm-1 and 3228 cm-1 are due to the stretching vibrations of O-H group. The peak at 2930 cm-1 and 1407 cm-1 are due to the stretching vibration of C-H group. The peak at 2174 cm-1 corresponds to the stretching vibration of C-O bond. The peaks at 1632 cm-1 and 1226 cm-1 are due to the C=O group. The peak at 1114 cm-1 corresponds to the stretching vibrations of C-N group attributed to aliphatic amines. The peak at 694 cm-1 corresponds to the characteristic peak of iron oxide nanoparticles [8]. The FTIR spectrum of FeNPs-GM confirms the iron oxide nanoparticles are coated by the naturally synthesized polysaccharide, galactomannan.
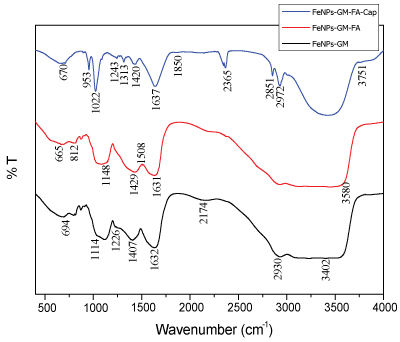 Figure 8: FT-IR spectrum of galactomannan coated iron oxide nanoparticles (Black), folic acid modified galactomannan coated iron oxide nanoparticles (Red) and folic acid modified galactomannan-coated iron oxide nanoparticles conjugated with capecitabine (Blue).
View Figure 8
Figure 8: FT-IR spectrum of galactomannan coated iron oxide nanoparticles (Black), folic acid modified galactomannan coated iron oxide nanoparticles (Red) and folic acid modified galactomannan-coated iron oxide nanoparticles conjugated with capecitabine (Blue).
View Figure 8
The FTIR spectrum of FeNPs-GM-FA shows a peak at 3580 cm-1 , which corresponds to the stretching vibrations of O-H group attributed to isourea. The sharp peak at 1631 cm-1 indicates the presence of C=O group. The sharp peak at 1508 cm-1 is the characteristic peak of C-C group attributed to the stretching vibration of aromatic C=C. The peaks at 1429 cm-1 and 1148 cm-1 are due to the presence of C-O group attributed to alcohol and carboxylic acid respectively. The peak at 1082 cm-1 corresponds to the stretching vibration of C-N group attributed to aliphatic amines. The weak absorption band at 812 cm-1 is due to the vibration of C-H bondoscillations of β-mannopyranose residues of galactomannan [9]. The peak at 665 cm-1 indicates the presence of magnetite [10]. The formation of new peaks at 1508, 1429, 1148 and 812 cm-1 corresponding to aromatic C-C, C-O, C-O of alcohol and aromatic C-H groups respectively, indicate the presence of folic acid.
In the FTIR spectrum of FeNPs-GM-FA-Cap, the peak at 3751 cm-1 corresponds to the O-H group. The Sharp peaks at 2927 and 2851 cm-1 corresponds to the stretching vibration of C-H bond of alkyl group. The strong peak at 2365, 1420 and 1243 cm-1 are due to the presence of C-O group. The peak at 1850 cm-1 is the characteristic peak of carbonyl group of esters. The strong peak at 1637 cm-1 is due to the presence of C=O group attributed to CONH2 group [11]. The peak at 1313 and 1022 cm-1 is due to the presence of C-N bond in aromatic amine and aliphatic amine respectively. The peak at 953 cm-1 is due to the stretching vibration of O-H bond attributed to carboxylic acid. The characteristic peak of Fe-O bond at 670 cm-1 indicates the presence of iron oxide nanoparticles [12]. When compared to the FT-IR spectrum of FeNPS-GM-FA and FeNPs-GM-FA-Cap, the shifts in bands from 2930 to 2927 cm-1 , 1632 to 1637 cm-1 and 1429 to 1420 cm-1 attributed to C-H, C=O and C-C group respectively, indicates that these groups are attached to the drug. The formation of new peaks at 2851, 2365, 1850, 1420, 1313, 1243 and 1022 cm-1 indicates the presence of capecitabine and the disappearance of peaks at 1508, 1148 and 812 cm-1 corresponding to aromatic C-C, C-O and aromatic C-H group respectively in FT-IR spectrum of FeNPs-GM -FA-Cap indicates that the corresponding groups are involved in the formation of FeNPS-GM -FA-Cap complex.
The extent of cytotoxicity from every single concentration of iron oxide nanoparticle was quantified as a percentage of cell viability including the absorbance values obtained as shown in the Figure 9. The percentages of cell viability above 80% are considered as non-cytotoxic; within 80%-60% are weak; 60%-40% are moderate and below 40% are strong cytotoxic [13]. As observed in the Figure 9, all the percentages were high and consequently, the iron oxide nanoparticles were non-toxic at concentrations even up to 100 μg/ml. The viability range was within 83% to 71%. Thus, the in-vitro cytotoxicity studies clarified that the synthesized iron oxide nanoparticles did not have any toxic effect to the Hep2 cell line at the concentrations tested in the MTT assay. The prepared nanoparticles are well tolerated by Hep2 cells, making it suitable for various biological applications.
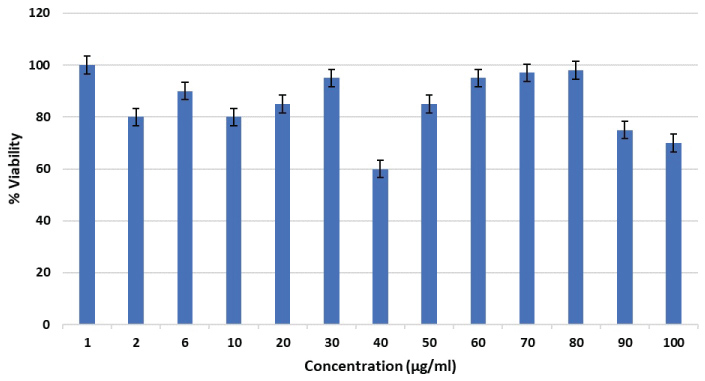 Figure 9: Effect of various concentrations of iron oxide nanoparticles on HEp2 cell viability.
View Figure 9
Figure 9: Effect of various concentrations of iron oxide nanoparticles on HEp2 cell viability.
View Figure 9
Recently investigations on iron oxides nanoparticles were increased in the field of nanosized magnetic particles (mostly maghemite, g-Fe2O3, or magnetite, Fe3O4, single domains of about 5-20 nm in diameter), among which magnetite is a very promising candidate since its biocompatibility has already proven [8].
Generally, in human cancer cell lines, the highest level of cytotoxicity was shown by 5-FU itself, followed by 50-dFUrd. Capecitabine and 50-dFCyd had shown mild cytotoxic activity only at high concentrations. The cytotoxicity of the intermediate metabolites 50-dFCyd and 50-dFUrd was suppressed by inhibitors of Cyd deaminase and dThdPase, respectively, revealing that these metabolites become active only after their conversion to 5-FU. Generally, Capecitabine is converted into 5-FU by dThdPase in tumour cells, should be much safer and more effective than 5-FU.
Figure 10 reveals the treatment of cells with iron nanoparticles. The synthesized iron oxide nanoparticles did not have any toxic effect to the HEp2 cell line at the concentrations tested in the MTT assay. The prepared nanoparticles are well tolerated by HEp2 cells, making it suitable for various biological applications.
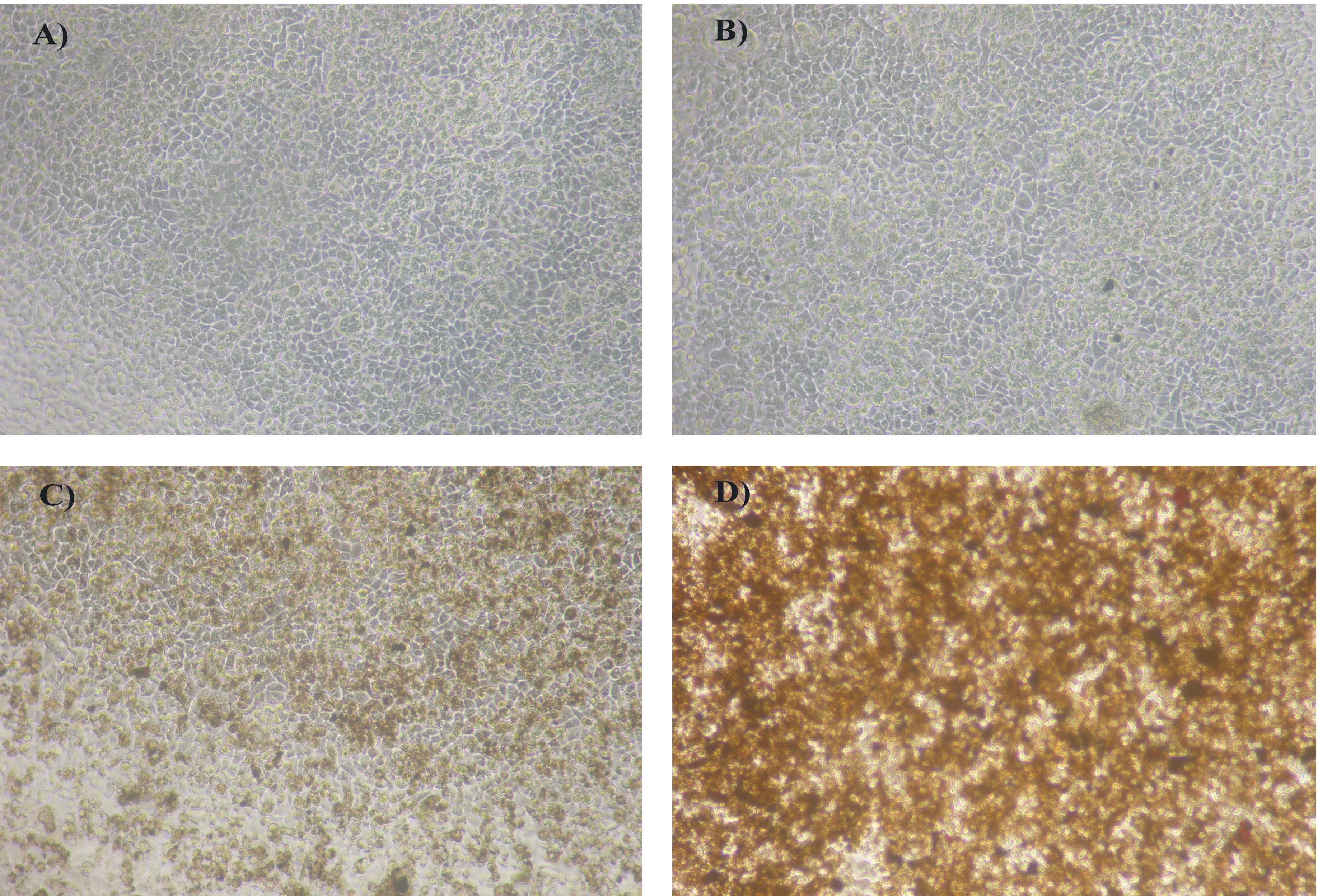 Figure 10: A) Control; B) Cells incubated with 2 μg/ml of iron oxide nanoparticles; C) Cells incubated with 40 μg/ml of iron oxide nanoparticles; D) Cells incubated with 100 μg/ml of iron oxide nanoparticles.
View Figure 10
Figure 10: A) Control; B) Cells incubated with 2 μg/ml of iron oxide nanoparticles; C) Cells incubated with 40 μg/ml of iron oxide nanoparticles; D) Cells incubated with 100 μg/ml of iron oxide nanoparticles.
View Figure 10
The drug release profile of Capecitabine from folic acid modified galactomannan functionalized iron oxide nanoparticles in PBS is given in Figure 11. The in-vitro drug release showed a slow, steady and controlled release of drug. Drug release profile shows a 30% release of capecitabine in 24 hours. Jin Hee Maeng, et al., observed similar results for doxorubicin loaded superparamagnetic iron oxide nanoparticles [14]. Drug release profile showed 32% release of drug in 72 hours. The drug is released gradually over a period. This shows that the drug is released in a sustained manner. The slow, steady and controlled release of drug shows that the drug molecules are strongly bound to the polymer. From the study, it is concluded that capecitabine loaded with folic acid modified GM functionalized iron oxide nanoparticles is an effective carrier for the sustained drug delivery.
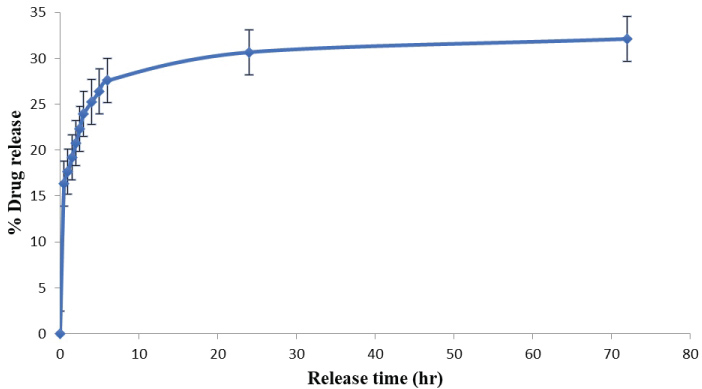 Figure 11: Drug release profile of capecitabine from folic acid modified GM functionalized iron oxide nanoparticles.
View Figure 11
Figure 11: Drug release profile of capecitabine from folic acid modified GM functionalized iron oxide nanoparticles.
View Figure 11
X-ray image of swiss wistar rat Pathological analysis of Hepatocellular carcinoma (HCC) is based on macroscopic and microscopic aspects that are highly diversified and associated with prognosis for some of them. After the administration of DEN carcinogen within one week they be tiny, discrete, translucent nodules on the capsular and surface cut surface of the remaining lobes. These results clearly indicate exposure of DEN chemical induces the changes in a few randomly located hepatocytes. The lesions continue to proliferate and become histologically in distinguishable from typical carcinogen induced hyperplastic liver nodules frequently described in the literature. Clinical evidence over several decade suggest that carcinogenesis is not a single step event but a gradual developmental process which may involves a series of sequential cellular alteration. Diethyl-nitrosamine (DEN) is known to induce liver cancer slowly with a single oral, intraperitoneal, intravenous administration. During the week following partial hepatocytes grow to become grossly visible as small translucent to grayish-white nodules, up to 1 mm in diameter, on the capsular and cut surface of the liver. The lesions are apparently induced by the DEN, for they are not formed in the absence of DEN pretreatment and they are numerically proportional to the initiating does of DEN [15].
HCC typically form soft masses with a heterogeneous macroscopic appearance, polychrome with foci of hemorrhage or necrosis. They could be single or multiple with a size ranging from less than 1 cm to over 30 cm. Usually on cirrhosis, the size of HCC is smaller compared to those developed in non-fibrotic liver [6]. On histology, the main hallmark of HCC is its resemblance to the normal liver both in its plate-like growth and its cytology [10]. HCC is usually a hyper-vascularized tumor showing different degrees of hepatocellular differentiation. As compared to healthy control, untreated group reveals impressive inductive effect of carcinogen non-liver. Free compound group shows regression but is associated with poorly preserved hepatocellular architecture. Healthy control groups show normal cords of hepatocytes and sinusoids, untreated group shows hypercellularity, cellular and nuclear polymorphism and overtly hyperchromatic larger nuclei with increase nuclear cytoplasmic ratio. Treated groups shows Hepatic cords and sinusoids appear very similar to healthy control. Treated group also shown increased cellularity, hyperchromatic nuclei, altered hepatocellular, contour, poorly maintained hepatocellular contour and dilated sinusoids.
Figure 12 reveals histological analysis of liver tissue sections from control group (1) animals revealed the normal architecture and cells with granulated cytoplasm and small uniform nuclei (Figure 12a). Group 2 DEN induced animals rat liver tissues revealed loss of their cell architecture, marked an ability to spread by intrahepatic veins, both hepatic and portal veins with significant tumor thrombi within portal vessels. Cytologically most of the tumor cells are slightly larger, have irregular shape of nuclei and numerous mitotic figures (Figure 12b). Group 3 FeNps capped with capecitabine treated animals showed normal architecture as well as some improved liver cell architecture indicating the therapeutic nature of capecitabine (Figure 12c). Because Capecitabine is a type of oral fluoropyrimidine carbamate, which is further converted into 5-fluorouracil (5-FU) molecule selectively in tumors through a cascade of three enzymes. This selectivity further supported by the FeNPs due their magnetic, hyperthermiea and EPR properties are make them effective usage in anticancer and drug delivery applications. A study reported that tissue localization of the three enzymes which are involving in the activation of capacitabine in humans, which was helpful for us to design the compound. Carboxylesterase was exclusively located in the liver and hepatoma, but not in other tumors and normal tissue adjacent to the tumors. There by it promoting the killing of cancer cells [16,17].
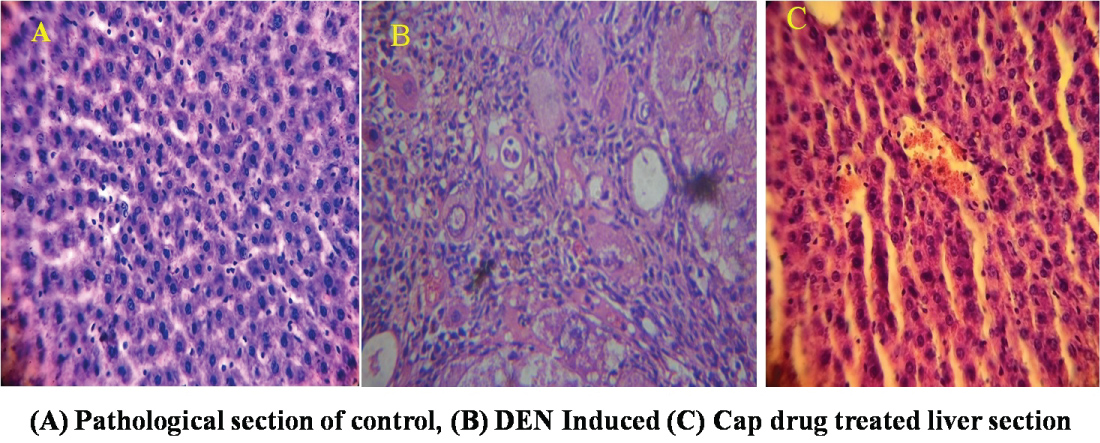 Figure 12: Histological evidence of liver tissue.
View Figure 12
Figure 12: Histological evidence of liver tissue.
View Figure 12
The main objective of nanosized drug delivery is accumulation of the drug molecules within tumors by EPR effect for the releasing of their therapeutic payloads. Hence, EPR effects are relatively potent, offering less than a 2-fold increase in delivery compared to critical normal organs. If the lifespan increases in circulation, which could help to extravasate nano drug into tumor through EPR effect. but at the same time, the drug can also extravasate into normal tissues albeit at a slower rate. Thus, methods that even temporarily increase the local EPR effect within the tumor are needed to improve the specific uptake of the drug within the tumor, thereby improving its therapeutic effect And FeNPs as such employed in hyperthermia applications. Iron magnetic nanoparticles used in the treatment of malignant tumours by hyperthermia (heat) method. In recent times, more advanced methods (hot water bath, pyrogens such as mixed bacterial toxins, perfusion heating, high-frequency radiation, magnetic fluid hyperthermia) were played vital role in heating and hopefully destroy, tumors [18]. Magnetic field induced hyperthermia, one of the important therapies for cancer treatment, means the exposition of cancer tissues to an alternating magnetic field. Usually magnetic field did not absorb by the alive tissues and can be applied to deep region in the living body. If the magnetic particles are subjected to a strong variable magnetic field, some heat is generated because of magnetic hysteresis loss. The total amount of heat generated completely depends on the nature and properties of magnetic material and of magnetic field parameters. Magnetic particles embedded in the tumor site and placed within an oscillating strong magnetic field will heat up to a temperature directly proportional to the magnetic properties of the material, the strength of the magnetic field, the frequency of oscillation and the cooling capacity of the blood flow in the tumor site. When compared to normal cells, cancer cells could be destroyed at higher temperature than 43.1 °C., but the normal cells can be surviving at higher temperatures. Heat could be generated applying an appropriate magnetic field. The size of the magnetite crystals is submicrometric, so the powders or bulk of these biomaterials have comparable properties. These materials are not only biocompatible, but also bioactive and could be useful for bone tumors [19].
Green synthesis of metallic nanoparticles is a novel approaches and alternative for chemical and physical method of preparation of nanoparticles. Mimosa pudica used to synthesize the iron oxide nanoparticles. Iron oxide nanoparticles were successfully functionalized with Galactomannan and FA. These nanoparticles containing FA can be used to successfully target tumor cells expressing folate receptors. Anticancer drug Capecitabine was attached to FeNPs-GM-FA molecule for functionalization for drug delivery and targeting, offering an initial burst release and later sustained drug release at the tumor site effectively. The positive outcome of cell culture studies on iron nanoparticles and histological studies of hepatic tissue treated with functionalized FeNPs confirm a potential application in cancer therapeutics.