Journal of Dermatology Research and Therapy
Targeting Myostatin Signaling in Skin Healing
Christoph Wallner*, Marcus Lehnhardt and Björn Behr
Department of Plastic Surgery, Burn Center, BG-University Hospital Bergmannsheil, Ruhr University Bochum, Germany
*Corresponding author:
Christoph Wallner, Department of Plastic Surgery, Burn Center, Hand Center, Sarcoma Reference Center, BG-University Hospital Bergmannsheil, Ruhr University Bochum, Germany, E-mail: c.wallner88@gmail.com
J Dermatol Res Ther, JDRT-2-014, (Volume 2, Issue 1), Short Review; ISSN: 2469-5750
Received: January 28, 2015 | Accepted: February 15, 2016 | Published: February 17, 2016
Citation: Wallner C, Lehnhardt M, Behr B (2016) Targeting Myostatin Signaling in Skin Healing. J Dermatol Res Ther 2:014. 10.23937/2469-5750/1510014
Copyright: © 2016 Wallner C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Myostatin is a protein well described for its role in decelerating muscle anabolism. Most studies targeting the Myostatin pathway were performed in muscle wasting diseases. Recent studies unveil a potential approach to interfere with the Myostatin pathway to facilitate wound healing. We therefore reviewed the present literature for aiming the Myostatin pathway as a potential treatment option in impaired skin healing. The inhibition of Myostatin may facilitate wound healing through different ways including reduced scarring, decreased inflammatory response and altered distribution of fat. Drugs targeting the Myostatin pathway are available for muscle wasting diseases but preclinical and clinical studies with those inhibitors are required to evaluate their potential in skin healing.
Keywords
Myostatin, Wound, Healing
Myostatin is known as Growth and Differentiation Factor 8 (GDF-8) and member of the TGF-β superfamily [1]. The protein is well described in muscle research for the negative regulatory effect of muscle growth and proposed as starting point of the treatment of e.g. muscle dystrophy Duchenne and other muscle wasting diseases [2,3]. A dramatic increase of muscle mass is observed in absence or alteration of Myostatin protein in cattle or dogs, resulting in the muscled Belgian Blue and a whippet breed respectively [4,5]. Different aspects of Myostatin decrease and inhibition shows an increase in muscle mass. On the other hand muscle wasting cancer cachexia shows upregulated Myostatin levels [6].
While research focused on myogenesis and muscle development, recent investigations uncovered a negative correlation of Myostatin and adult muscle regeneration [7]. In muscle regeneration, chemotaxis of macrophages is down regulated by Myostatin, while migration of fibroblasts is increased, resulting in more scarring [8]. Consequently, Myostatin-null mice show higher tissue regeneration and less fibrosis [7,9]. On the other hand Myostatin null mice expressed a reduced migration capacity and increased proliferation rateof keratinocytes. However this study unveiled decelerated wound healing but didn't point out the quality of the scar [10]. A study with full thickness burns in a rodent model exhibited a fourfold increase of Myostatin expression [11]. Skin compartments express Myostatin and its receptor Act RIIB, suggesting a potential target for Myostatin inhibition in skin healing [10,12]. Previous studies propose a therapy aiming at Myostatin expression, which might facilitate wound healing.
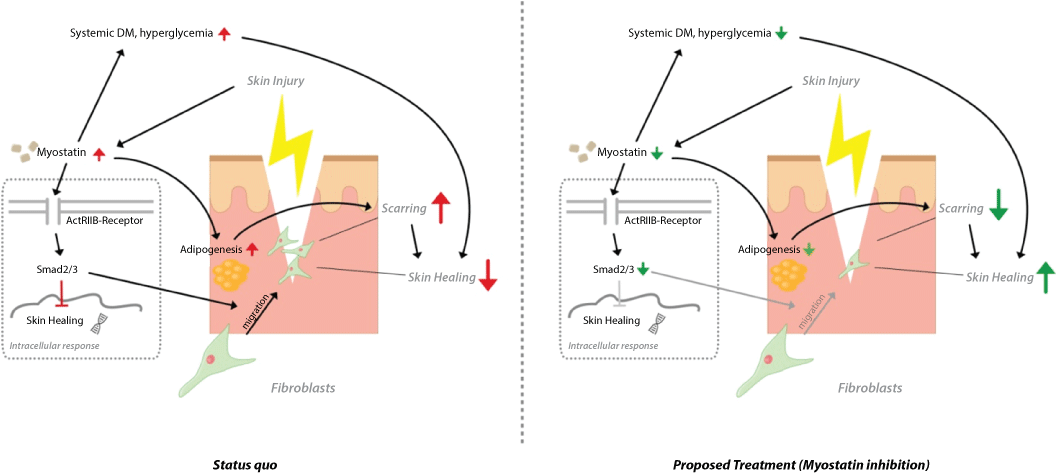
Reasons for compromised wound healing could be diabetes mellitus or peripheral arterial occlusive disease. Recent studies suggest a systemic elevation of Myostatin in diabetes mellitus, while inhibition improves systemic diabetes parameters. This might be caused by a decreased expression of Myostatin downstream target Smad3, which is described to play a role in diabetes pathogenesis [13]. Smad3 deficiency in mice protects against insulin resistance and type 2 diabetes during high-fat diet-induced obesity. Additionally to a metabolic upregulation Myostatin deletion prevents vascular deficits in obesity (Tan et al., 2012). Differentiation of embryonic fibroblasts to white fat tissue adipocytes is markedly reduced in Smad3 knockout mice. These mice present a dramatic reduction in adiposity as a result of decreased adipocyte number and size [14] (Figure 1).
.
Figure 1:Proposed pathological mechanism of impaired skin healing and potential targeting of Myostatin signalling.
(Left) Myostatin signaling leads to increased adipogenesis, deviated systemic diabetes parameters, fibroblast migration and in turn to augmented scarring and reduced skin sufficient healing. Taken together from [7,8,11,13,14]. DM stands for diabetes mellitus. (Right) The proposed treatment with Myostatin inhibition would facilitate wound healing with reduced scarring.
View Figure 1
Skin is a complex immunogenic organ and inflammation plays a major role in wound healing. Myostatin downstream target Smad3 deficient mice exhibit less inflammatory macrophage infiltration. Simultaneously TNF-α, IL-6 and MCP-1 are described to be down regulated in Smad3 knockout mice in white adipose tissue [14]. Ways to inhibit Myostatin may cause a reduced immunogenic response.
Different approaches of impeding Myostatin have been described. Amongst Myostatin propeptide, soluble activin receptor, Myostatin antibody (Stamulumab) and the follistatin-related proteins, Follistatin is utilized in the literature most frequently [15]. The Myostatin antibody is a recombinant human antibody intentionally designed to treat muscle dystrophy Duchenne by suppressing Myostatin binding to its target site. However, it was stopped in its phase I/II trial in 2008 [16]. Another study showed increased wound hydration, body weight and expression of fibromodulin and TGF-β3, both indicators for scarless healing [17].Clinical feasible approaches to inhibit Myostatin for facilitating wound healing might be local Follistatin (Myostatin inhibitor) application. Furthermore studies showed an improvement of systemic diabetes parameters by systemic application of Follistatin. Follistatin as drug for muscle wasting diseases is well described and might be the most promising starting point.
Taken together most studies suggested a potential treatment approach in impaired wound healing by inhibiting the Myostatin pathway. Furthermore Myostatin inhibition showed improved circumstances for a better wound healing with improvement of diabetic systemic parameters, reduction of scarring and alteration of fat distribution facilitating skin healing. Wound healing is a major economic challenge in the modern world. Effective strategies to overcome delayed or impaired skin healing would target this problem. Myostatin inhibition could be one way to improve diminished skin healing in different underlying diseases as diabetes mellitus or peripheral arterial occlusive disease. However further studies are demanded to evaluate the promising effects of Myostatin inhibition on skin healing.
References
-
McPherron AC, Lawler AM, Lee SJ (1997) Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387: 83-90.
-
Mendell JR, Sahenk Z, Malik V, Gomez AM, Flanigan KM, et al. (2015) A phase 1/2a follistatin gene therapy trial for becker muscular dystrophy. Mol Ther 23: 192-201.
-
Rodino-Klapac LR, Haidet AM, Kota J, Handy C, Kaspar BK, et al. (2009) Inhibition of myostatin with emphasis on follistatin as a therapy for muscle disease. Muscle Nerve 39: 283-296.
-
Kambadur R, Sharma M, Smith TP, Bass JJ (1997) Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res 7: 910-916.
-
Shelton GD, Engvall E (2007) Gross muscle hypertrophy in whippet dogs is caused by a mutation in the myostatin gene. Neuromuscul Disord 17: 721-722.
-
Jespersen J, Kjaer M, Schjerling P (2006) The possible role of myostatin in skeletal muscle atrophy and cachexia. Scand J Med Sci Sports 16: 74-82.
-
McCroskery S, Thomas M, Platt L, Hennebry A, Nishimura T, et al. (2005) Improved muscle healing through enhanced regeneration and reduced fibrosis in myostatin-null mice. J Cell Sci 118: 3531-3541.
-
Siriett V, Salerno MS, Berry C, Nicholas G, Bower R, et al. (2007) Antagonism of myostatin enhances muscle regeneration during sarcopenia. Mol Ther 15: 1463-1470.
-
Li ZB, Kollias HD, Wagner KR (2008) Myostatin directly regulates skeletal muscle fibrosis. J Biol Chem 283: 19371-19378.
-
Zhang C, Tan CK, McFarlane C, Sharma M, Tan NS, et al. (2012) Myostatin-null mice exhibit delayed skin wound healing through the blockade of transforming growth factor-β signaling by decorin. Am J Physiol Cell Physiol 302: C1213-1225.
-
Lang CH, Silvis C, Nystrom G, Frost RA (2001) Regulation of myostatin by glucocorticoids after thermal injury. FASEB J 15: 1807-1809.
-
Hübner G, Brauchle M, Smola H, Madlener M, Fässler R, et al. (1996). Differential regulation of pro-inflammatory cytokines during wound healing in normal and glucocorticoid-treated mice. Cytokine 8: 548-556.
-
Guo T, Bond ND, Jou W, Gavrilova O, Portas J, et al. (2012) Myostatin inhibition prevents diabetes and hyperphagia in a mouse model of lipodystrophy. Diabetes 61: 2414-2423.
-
Tan CK, Chong HC, Tan EH, Tan NS (2012) Getting 'Smad' about obesity and diabetes. Nutr Diabetes 2: e29.
-
Patel K, Amthor H (2005) The function of Myostatin and strategies of Myostatin blockade-new hope for therapies aimed at promoting growth of skeletal muscle. Neuromuscul Disord 15: 117-126.
-
Wagner KR, Fleckenstein JL, Amato AA, Barohn RJ, Bushby K, et al. (2008) A phase I/IItrial of MYO-029 in adult subjects with muscular dystrophy. Ann Neurol 63: 561-571.
-
Fieten DJ (2009) The Effect of a Myostatin Antagonist on the Healing of Burn Wounds in Skin. The University of Waikato.





