Diabetic nephropathy remains the most common cause of end-stage renal disease (ESRD) worldwide. Microalbuminuria is the first clinical sign of renal dysfunction in patients with diabetes mellitus. A urine total protein: creatinine ratio (TPCR) is a convenient and inexpensive measure of proteinuria and could be used to predict the presence of microalbuminuria in diabetic patients.
The aim of this study was to assess the prevalence and associated risk factors of proteinuria among type-2 diabetic patients in Dhamar governorate, Yemen.
This was a cross sectional study, spot urine TPCR was performed on 200 diabetic patients who attended the diabetic clinic at Dhamar General Hospital and Al-Wahda Teaching Hospital from March 2016 to June 2016.
This study indicated higher prevalence of pathological proteinuria (TPCR ≥ 150 mg/g of creatinine) 64% of the study population. Estimated urine albumin-to-creatinine ratio (ACR) revealed that 49% of the diabetic patients had microalbuminuria (ACR 30-300 mg/g) and 25.5% was estimated as macroalbuminuria (ACR > 300 mg/g). Multivariate analysis revealed that the Hypertension (Ad OR: 3.44; % CI: 1.57-7.55; p: 0.002), duration of diabetes 5-10 years (Ad OR: 5.55; % CI: 2.22-13.89; p: 0.000) and beyond 10 years (Ad OR: 8.88; % CI: 3.04-24.77; p: 0.000) and obesity (Ad OR: 0.15; % CI: 0.03-0.69; p: 0.015) were associated with a progressively greater likelihood of pathological proteinuria.
The present study revealed high prevalence of nephropathy among Type-2 diabetic patients. Hypertension, duration of diabetes and obesity are the risk factors associated with diabetic proteinuria. Screening for TPCR and estimation of microalbuminuria allow early identification of nephropathy which help in implementing effective interventions to manage the risk factors and prevent the serious diabetic complications.
Proteinuria, Prevalence, Diabetes, Yemen
Diabetic nephropathy is a significant cause of chronic kidney disease and end-stage renal failure globally. The incidence of end-stage renal failure in patients with type-2 diabetes mellitus (T2DM) has dramatically increased in recent years. Diabetes was reported to be the primary cause of end-stage renal disease (ESRD) in about 60% of patients in Malaysia, Mexico, Singapore and 40%-60% in the US, Israel, Korea and Taiwan in 2009-2011 [1].
Diabetic nephropathy usually manifests after 10 years duration of type 1 diabetes mellitus (T1DM), but may be present at diagnosis of T2DM. Screening for microalbuminuria should be initiated five years after diagnosis of T1DM and at diagnosis of T2DM [2]. Diabetic nephropathy has been didactically categorized into stages based on the values of urine albumin/creatinine ratio (ACR): microalbuminuria (ACR = 30-300 mg/g of creatinine) and macroalbuminuria (ACR > 300 mg/g of creatinine) [3].
Because of the increasing worldwide prevalence T2DM and the serious consequences of this disease on global health. In situations where specific urine albumin measurements are not available. Another alternative is to use a qualitative test for proteinuria (dipstick) or a quantitative measurement of protein in a spot urine sample [4,5]. Several studies reported that the upper limit of normal for urine total protein excretion for adults is generally 150 to 200 mg/day [6,7] and there appears to be general agreement that values of 300 mg/day or higher are clearly abnormal [8,9].
The urine TPCR is an inexpensive laboratory test and can be used to determine if excess levels of protein are present in the urine. Although microalbuminuria is preferred as a marker over TPCR, the cost of measuring albumin may limit its use in some countries [10,11]. In addition to the low cost of TPCR a previous study concluded that ACR and TPCR were comparable in predicting ESRD or mortality [12]. Furthermore, it has been reported that TPCR is a more sensitive screening test than ACR to predict clinically relevant proteinuria and highly correlated with 24-h urine protein than ACR [13].
The Japanese Society of Nephrology and the Kidney Disease: Improving Global Outcomes Chronic Kidney Disease (KDIGO CKD) guidelines state that a urine TPCR of 150 mg/g of creatinine is equal to an ACR of 30 mg/g of creatinine [14]. Furthermore, a previous study in a diabetic population, reported a significant positive correlation between the TPCR and the urine ACR (r = 0.95) and that the TPCR could predict the presence of microalbuminuria in more than 90% of diabetic patients with sensitivity and a specificity of 90.8% and 91.9%, respectively, for the detection of albuminuria [15].
In Yemen the overall prevalence of T2DM was reported 4.6% (7.4% in males and 2% in females) [16]. A previous study carried out at Sana'a City (Yemen), found that the prevalence of diabetic nephropathy is 33.5% among T2DM patients [17]. However, there is still a need more studies to evaluate the broaden of the daises in Yemen. In Dhamar governorate, Yemen, to the best of our knowledge, there is no study evaluating the prevalence of diabetic proteinuria and nephropathy among patients with T2DM. We designed this study to estimate the prevalence of diabetic proteinuria and to identify possible risk factors for the development of proteinuria among subjects with T2DM. On its basis; we also undertake further research on the actual prevalence among similar risk groups in the country.
The study was conducted in Dhamar governorate, which is located 100 km to the south of the capital Sana'a with estimated population of around one and a half million. Dhamar governorate enclosed by two public hospitals; Dhamar General Hospital and Al-Wahda Teaching Hospital which are the main referred hospitals.
This was a cross-section study carried out at Dharma General Hospital and Al-Wahda Teaching Hospital. A total of 200 T2DM individuals (115 men and 85 women with mean age 53 ± 12 and 52 ± 12 respectively) were randomly selected from those who had visited diabetic outpatient clinic during March 2016 to June 2016. All patients enrolled in this study were already diagnosed with T2DM based on American diabetic association criteria.
Pretested questionnaires were used to obtain a medical history including the medical treatment being given duration of DM, hypertension and others risk factors. The duration of DM was defined as the time interval in years between the date of first-time diagnosis of DM and the date of present evaluation. A hypertensive diabetic patient was defined as a person who showed a reading of more than 140 mmHg systolic blood pressure and/or > 90 mmHg diastolic blood pressure, or a patient was already taking medicine to control hypertension even though blood pressure measurements were within the normal range.
Pathological proteinuria was defined using spot urine TPCR cut off 150 mg/g of creatinine [14]. ACR was calculated from TPCR by means of equation extracted from the correlation between the urine ACR and the urine TPCR which stated that: Ln ACR = 1.326 X Ln TPCR - 2.64 [15]. The types of albuminuria were defined as: normoalbuminuria (ACR < 30 mg/g creatinine), microalbuminuria (ACR 30-300 mg/g creatinine) and macroalbuminuria (> 300 mg/g creatinine) [3].
Patients with known renal failure, heart failure, and acute febrile illness were excluded from the study. Microscopic examination of urine sediments was use to exclude diabetic patients with urinary tract infection and hematuria.
This study was approved by ethical and research committee of Thamar University Faculty of Medicine and Health Sciences. We obtained written consents from the patients to participate in this study.
Data were analyzed using Statistical Package for Social Science (SPSS) version 23. Continuous va¬riables were categorized according to standard criterions and presented as percen¬tages. Univariate analysis and Multiple logistic regression analyses was done using Pathological Proteinuria (TPCR ≥ 150 mg/g of creatinine) as the dependent variable and the variables tested included age, gender, blood pressure, duration of diabetes, BMI, family history, physical activity, antihyperglycemic agents, smoking Qat chewing, and education. A P-value of < 0.05 was considered to be statistically significant.
The selected patients were instructed to collect untimed spot urine samples. The urine samples were collected at room temperature, without adding any preservatives. Immediately after their collection, the urine samples were analyzed for protein and creatinine. Total urine protein was quantitively estimated by colorimetric procedure using a pyrogallol red molybdate assay [18]. Urinary creatinine concentration was done by the modified Jaffe's method [19]. The urine protein: creatinine ratio was calculated by dividing the urine protein concentration (mg/dl) by urine creatinine concentration (g/dl). The ACR was calculated from urine TPCR based on the formula: Ln ACR = 1.326 X Ln TPCR - 2.64 [15].
The general characteristics of the study population are shown in Table 1. Of 200 type 2 diabetic patients in this study, there were 115 (57.5%) male and 85 (42.5%) female with mean age 53 ± 12 and 52 ± 12 years respectively. Of the patients 32% had hypertension, 56% had diabetic duration of 5-10 years and 29% > 10 years. 68.5% of the study population were using oral antihyperglycemic therapy, 24% were using insulin and 7.5% of the diabetic patients were not using any antihyperglycemic medicine. About half of the study population were obese, 43% had a family history of T2DM, 15% physically active, 27.2% smoker and 64.5 were uneducated.
Table 1: General characteristics of the study population (N = 200). View Table 1
The overall prevalence of pathological proteinuria (TPCR ≥ 150 mg/g creatinine) was found in 132 (66%) of the study population (Figure 1).
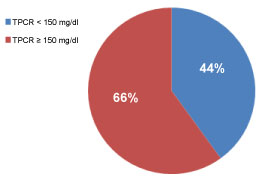 Figure 1: Prevalence of pathological proteinuria (TPCR ≥ 150 mg/g creatinine TPCR); TPCR: Total protein to creatinine ratio. View Figure 1
Figure 1: Prevalence of pathological proteinuria (TPCR ≥ 150 mg/g creatinine TPCR); TPCR: Total protein to creatinine ratio. View Figure 1
The prevalence of albuminuria is shown in Figure 2. ACR was calculated from TPCR by formula; Ln ACR = 1.326 X Ln TPCR - 2.64 [13]. In total of 200 T2DM patients participated in this study, 25.5% was normal compared to 49% and 25.5% were estimated as microalbuminuria (ACR 30-300 mg/g) and macroalbuminuria (ACR > 300 mg/g) respectively.
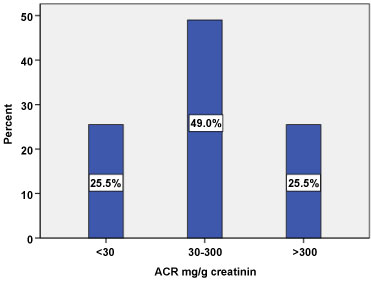 Figure 2: Estimated Albuminuria categories among type 2 diabetic patients (N = 200). View Figure 2
Figure 2: Estimated Albuminuria categories among type 2 diabetic patients (N = 200). View Figure 2
The frequency distribution of pathological proteinuria based on the clinical and demographic features of the study population are shown in Table 2. The prevalence of pathological proteinuria was significantly high in patients with hypertension (81.3%), diabetic duration > 10 years (75.9%), diabetic duration 5-10 years (69.9%), obese patients (69.1%) and with those patients were taking oral antihyperglycemic medicine (59.9%), and insulin (85.4). However, there were no significant differences between groups with respect to age, gender, family history of diabetes, physical activity, smoking, Qat chewing, and education.
Table 2: Factors associated with pathological proteinuria among type 2 diabetes (N = 200). View Table 2
Univariate analysis showed the demographic and clinical characteristics that were associated with proteinuria. Diabetic patients with hypertension were significantly more likely to exhibit Proteinuria (OR = 3.033; % CI = 1.5-6.2; p = 0.002) compare to diabetic patients without hypertension. Subjects with diabetic duration more than 5 years were significantly more likely to display proteinuria. Diabetic patients with duration of diabetic between 5-10 years were significantly more likelihood of proteinuria (OR = 4.59, % CI = 1.94-10.84, p = 0.00) and the category beyond 10 years of diabetic duration was observed to be associated with a progressively greater likelihood of proteinuria (OR = 6.27, % CI = 2.38-16.55, p = 0.000). The other demographic and clinical characteristics were not significant predictors of the presence of proteinuria in this study population.
Multivariate analysis (Table 3) adjusted for age, gender, physical activity and smoking showed significant association between pathological proteinuria as dependent variable and potential risk factors. The results indicated that hypertension (Ad OR: 3.44; % CI: 1.57-7.55; p: 0.002), duration of diabetes 5-10 years (Ad OR: 5.55; % CI: 2.22-13.89; p: 0.000) and beyond 10 years (Ad OR: 8.88; % CI: 3.04-24.77; p: 0.000) and obesity (Ad OR: 0.15; % CI: 0.03-0.69; p: 0.015) were significant risk factors associated with pathological proteinuria.
Table 3: Multivariate analysis for the potential risk factors (N = 200). View Table 3
Proteinuria is a well-recognized predictor of ESRD and all-cause mortality rates, as well as cardiovascular mortality rates [20,21]. Between 35% and 57% of T1DM and 25% and 46% of T2DM patients with long-lasting diabetes develop clinically detectable nephropathy, indicated by proteinuria and/or renal insufficiency [22,23].
The major findings of this cross-sectional study are that prevalence of pathological proteinuria (TPCR ≥ 150 mg/g creatinine) among type 2 diabetic patient is 66% which is similar to that in other comparable studies [24]. The frequency of pathological proteinuria increases after 5 years of diabetic duration. The highest prevalence of diabetic related pathological proteinuria was found in hypertension (81%), diabetic duration > 10 years (75%) and patients receiving insulin (85%). Duration of diabetes and hypertension have been shown to be risk factors for nephropathy by almost all earlier studies [25-27]. The high prevalence of proteinuria in patients receiving insulin at the time of this study could be explained by previous uncontrolled glycemic state which has led them to use insulin as the final choice of treatment.
Estimated microalbuminuria (ACR 30-300 mg/g) and macroalbuminuria (ACR > 300 mg/g) are 49% and 25.5% respectively. These rates were clearly higher than the equivalent rates reported in Yemen by study carried out in Al-Kuwait University Hospital which determine that the overall prevalence of diabetic nephropathy among type 2-diabetic patients was 33.6% (21.2% had microalbuminuria and 12.4% had macroalbuminuria) [17]. The rise in the prevalence of diabetic nephropathy in our study might be a result of apparent deficiency in the primary health care during the last five years in Yemen because of the war and political conflicts. Although the prevalence of microalbuminuria was unexpectedly high, this is consistent with studies in Saudi Arabia (45.6%) [28], in Sudan 44% [29] and in Kuwait 58.2% [30].
According to previous studies, the prevalence of macroalbuminuria was 12.8% in Egypt [31]), 12.7% in Taiwan, 11.2% in Thailand, 16% in Italy as well as Sweden and 9% in Germany [25]. These variations in the prevalence rate of proteinuria can be attributed to differences in several factors such as; study design, source of study population, diagnostic criteria, as well as the methods of measurement of proteinuria and urine collection, diabetic duration, diabetic treatment, and presence of hypertension. The high prevalence of microalbuminuria and macroalbuminuria in patients with T2DM is attributed to the presence of diabetes many years before it is actually diagnosed.
The demographic and clinical characteristics that were associated with proteinuria were presented in univariate analysis (Table 2). Our study indicated that hypertension diabetic duration more than 5 and 10 years, obesity and untreated patients were associated with increased diabetic proteinuria.
Multivariate analysis identified the potential risk factors associated with developing pathological proteinuria in T2DM patients which are; Hypertension, duration of diabetes and obesity. Diabetic patients with hypertension were found to be 3.4 times greater risk to develop pathological proteinuria (Ad OR: 3.44; % CI: 1.57-7.55; p: 0.002). There is evidence that microalbuminuria and proteinuria correlate more closely with abnormal 24-h Blood Pressure measurements in both diabetic and non-diabetic patients [25]. Diabetes duration was identified to be the most important risk factor for developing nephropathy especially ≥ 15 years in different ethnic groups [26,27]. The present study present confirms the above findings, diabetic patients with duration of > 10 years were identified to be more likely to develop pathological proteinuria (Ad OR: 8.88; % CI: 3.04-24.77; p: 0.000) and those with duration between 5-10 years (Ad OR: 5.55; % CI: 2.22-13.89; p: 0.000) compared to those with diabetes in a duration less than 5 years. Obesity has been identified as one of the risk factors associated with increased proteinuria [32,33], we found that obese patients were significantly associated with higher prevalence of pathological proteinuria (Ad OR: 0.15; % CI: 0.03-0.69; p: 0.015).
In this study population, age, gender, family history of diabetes, smoking and education were not observed to be significantly associated with proteinuria. It seems that age is not important and what is important is the duration of diabetes [27,32]. Although many studies showed a disagreement concerning the gender as a risk factor for the development of renal disease in DM [33,34].
Several limitations of this study must be acknowledged. First, glycemic control status is a potential factor associated with chronic diabetic complications including nephropathy. A marker of glycemic control such as glycated hemoglobin was not included in the analysis because it was not available. Furthermore, the association of Glomerular Filtration Rate and proteinuria was not evaluated in this study. Second, the study sample size was limited, and the results may not reflect the diabetic population in general. The extent to which the findings of this study can be generalized to the general diabetic population requires further investigation. Third, ACR was not directly estimated, instead it was calculated from TPCR based on the formula established in other study. Despite these limitations, the current study highlighted the degree of diabetic nephropathy in type 2 diabetic patient which required an effective action.
In conclusion, this study revealed that the prevalence of diabetic proteinuria found to be high (66%) in our study population. The prevalence of diabetic nephropathy based on estimated ACR was 74.5% (49% microalbuminuria and 25.5% macroalbuminuria). The risk factors identified in the present study are long duration of diabetes, hypertension, and obesity. This is seriously alarming the health professionals to implement effective intervention strategy for better control of these risk factors in type 2 diabetic Patients which may lower their risk for diabetic nephropathy.
The authors would like to express their sincere thanks to graduated students of Faculty of Medicine and Health Sciences, Thamar University, Dr Ibraheem abu Hadi, Ayman Alwagih, Ibraheem Alhindi, Ahlam Alhiar, Reham Alhitar, Musa Alssiad for their participation in this study. The authors extend their appreciation to Dr. Faheem Al-Mujahid for his contribution in this work. This work was partially supported by Faculty of Medicine and Health Sciences, Thamar University. We wish to express our sincere gratitude for the dean of the faculty prof. Amat Al-Khaleq Obad Mehrass and research section in our faculty for their support, without which it would be impossible to run this work.
The authors have no conflict of interest to declare.