Neuroendocrine breast carcinomas (NEBC) are rarely malignant with a frequency of less than 0.1-0.3% of all breast tumors. The actual incidence of NEBC in BC (Breast Cancer) populations being still largely unknown due to the lack of a clear cut diagnostic criteria. In 2003, the World Health Organization (WHO) Classification of Tumors of the Breast and Female Genital Organs definitely established that the immunohistochemical expression of NE markers is the unique requirement for NEBC diagnosis [1] in more than 50% of the tumor cell population. In the 2012 WHO Classification of the Tumors of the Breast these entities were collected in another chapter, among the special sub-types: Carcinomas with neuroendocrine features, which encompass the categories of Neuroendocrine tumors which are well differentiated, Neuroendocrine carcinoma which is a poorly differentiated/small cell carcinoma and Invasive breast carcinoma with neuroendocrine differentiation.
We would like to report the case of a 67-year-old woman with a rare neuroendocrine well differentiated breast cancer detected accidentally during a PET performed as a follow up in the treatment of a bone plasmacitoma. The result of histological examination was well differentiated primary neuroendocrine tumor of the breast with CKPan+, SYn+; CK7- and CK5-; ER+, Pr+, HER2- and ki67 < 5% [1].
The prognosis of NECB is not different from other invasive breast carcinomas and the most important prognostic factor is the tumor grade (G). However, there is no standard treatment and patients should be treated similarly to patients with invasive ductal carcinoma, NOS (Not Otherwise Specified), the choice of therapy depending on the size of the tumor, the degree of differentiation, the clinical stage, and the hormonal status.
NEBC: Neuroendocrine Breast Carcinoma; BC: Breast Cancer; NOS: Not Otherwise Specified
Neuroendocrine carcinomas are rarely malignant tumors which are mostly located in the lungs and the gastrointestinal tract. Primary location of such tumors in the breast is extremely rare, corresponding to less than 0.1-0.3% of all breast tumors and less than 1-3% of all neuroendocrine tumors. In fact, the true incidence of the disease is difficult to assess, because the immunohistochemistry neuroendocrine markers are not routinely used in breast tumors [2].
NECB was originally reported by Feyrter and Hartmann [3] in 1963 as an invasive breast cancer morphologically similar to intestinal carcinoids. In 1977, Cubilla and Woodruff [4] described eight other cases of breast cancer with a carcinoid growth pattern, providing for the first time histopathological classification together with a clinical and prognostic analysis of NECB.
Focal neuroendocrine differentiation can be found in different histological types of breast carcinoma including in situ and invasive ductal or invasive lobular ones. Up to 30% of invasive carcinomas and particularly mucinous carcinomas may be detected by means of histochemical and immunohistochemical analysis.
According to WHO, the neuroendocrine carcinoma is a tumor with positive immunoreactivity to neuroendocrine markers, synaptophysin and/or chromogranin, in at least 50% of tumor cells [5].
In 2012, WHO classified these tumors into three categories:
(1) Well differentiated neuroendocrine tumor;
(2) Poorly differentiated/small cell carcinoma. The most commontumor, which is CK7+ and Ck20, is morfologically similar to the small lung cell carcinoma which is negative for both markers;
3) Invasive breast carcinoma with neuroendocrine differentiation Er+, PR+ [6].
Neuroendocrine differentiation is an independent adverse prognostic factor for the first two specific categories and the overall survival. The average of onset is 64-years-old [7].
Sapino, et al. describe 5 histological subtypes: Solid, alveolar, small cell, solid papillary and mucinous [8].
Adjuvant treatment should follow the same medical plan as the other types of invasive breast cancer. An accurate diagnosis of NECB is also important in the metastatic setting, in which a multimodality approach including specific therapies such as peptide receptor and radionuclide therapy can be considered [20].
We are here describing the case of a 67-year-old woman with a well-differentiated plasmocellular plasmacytoma which was diagnosed in 2011; with restriction k, FISH positive for del17p13.
Because of that she underwent chemotherapy according to the VTD scheme (Velcade, Thalidomide, Dexamethasone) + zometa, with complete response to the treatment.
In 2018 the patient complained of widespread osteoarticular pains for which a BOM was carried out, which was negative. After that, a CT total body showed a 16-mm diameter swelling in the upper inner quadrant of the left breast. She also underwent a PET 18-FDG that showed an increase of hyper metabolism of 18 FDG localized in the left breast and also in the left iliac branch. For this reason a PET Ga DOTATOC was performed to exclude a plasmacitoma origin or a primary lesion. The PET confirmed aniliac lesion in relation to a plasmacitoma. A tru-cut biopsy instead showed a ductal carcinoma in the left breast. A quadrantectomy and sentinel limpho node biopsy were performed. The histological result was a well differentiated primary neuroendocrine cancer of the breast: pT1c N0, G1, 1.7 cm with CKPan+, SYn+; CK7- and CK5-; ER 90%, Pr 80%, Herb negative and ki67 < 5%. The sentinel node biopsy was negative. Since according to literature there is no standard treatment patients should be treated similarly to patients with ductal invasive carcinoma. Consequently, she underwent radiotheraphy and hormonal therapy.
The histogenesis of NECB has not been clearly defined yet. One of the theories suggests the development of NECB from neuroendocrine cells which are constitutively present in the breast, but these cells have not been consistently found in normal breast tissue [9]. Furthermore, neuroendocrine cell hyperplasia or in situ neuroendocrine carcinoma that would support a progression from noninvasive to invasive neuroendocrine tumors have been rarely reported [10]. According to another theory, NECB would instead result from an early divergent differentiation of breast cancer stem cells into both neuroendocrine and epithelial lines [11]. This hypothesis is consistent with the observation that NECB is always a mixed tumor including endocrine and exocrine cells, and it is also consistent with molecular studies showing that in NECB the neuroendocrine cells are clonally related to the intraductal component of the tumor [12].
NECB is often positive for hormone receptors, whereas HER2 is almost always negative. Accordingly, the gene expression profile analysis has revealed that NECB belongs to the Luminal A subtype [13]. A luminal B phenotype, defined by immunohistochemistry as hormone receptor-positive tumor with high proliferation index (i.e., Ki67 > 14%), accounts for approximately 50% of NECB [14]. However, there are also reports of HER2-positive NECB [15] and small-cell carcinoma of the breast with basal-like characteristics.
The tumor consists of densely cellular, solid nests (prevalent aspect in our case) and trabeculae of round cells of small-medium size, with abundant clear cytoplasm, separated by delicate fibrovascular stroma. The cells have round, regular nuclei and show some palizading arrangement at the periphery of the nests.
The immunophenotype was coherent with the diagnosis, showing bright positivity for synaptophysin, while CK5 and CK7 were negative.
Estrogenic and progestinic receptors showed diffuse positivity as well, with low proliferative fraction (Ki67/Mib1 < 5%) and no amplification of HER2 gene (data not shown) (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9 and Figure 10).
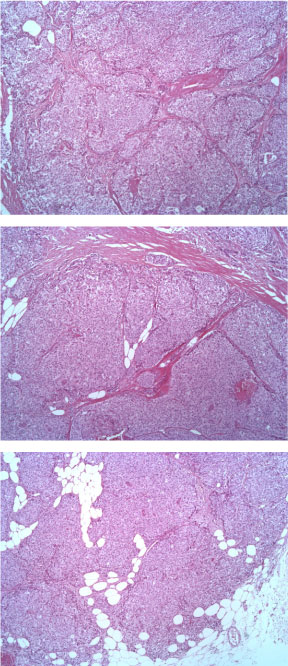 Figure 1: EE ingrandimento 5X. View Figure 1
Figure 1: EE ingrandimento 5X. View Figure 1
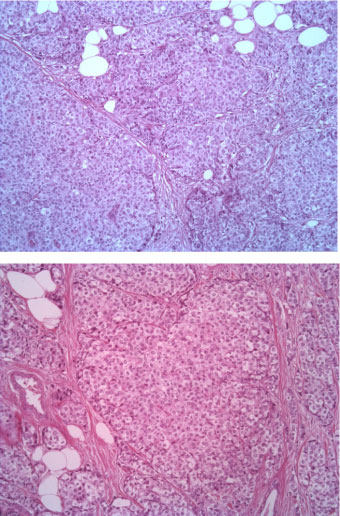 Figure 2: EE 10X. View Figure 2
Figure 2: EE 10X. View Figure 2
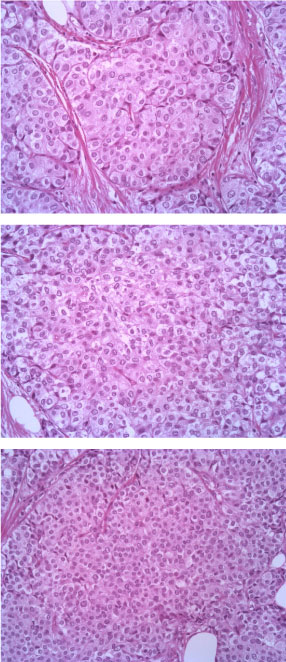 Figure 3: EE 20X. View Figure 3
Figure 3: EE 20X. View Figure 3
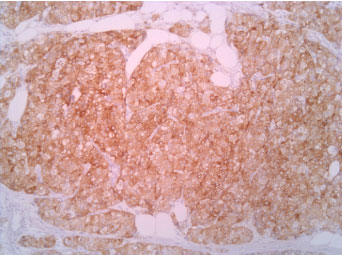 Figure 4: Synaptophisyn 10X. View Figure 4
Figure 4: Synaptophisyn 10X. View Figure 4
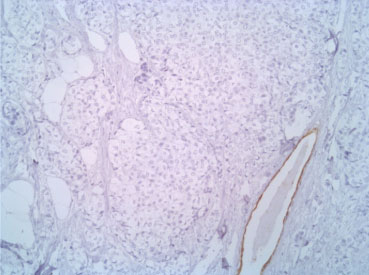 Figure 5: CK7 10X. View Figure 5
Figure 5: CK7 10X. View Figure 5
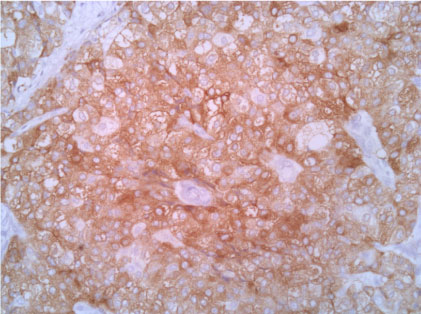 Figure 6: Synaptophisyn 20X. View Figure 6
Figure 6: Synaptophisyn 20X. View Figure 6
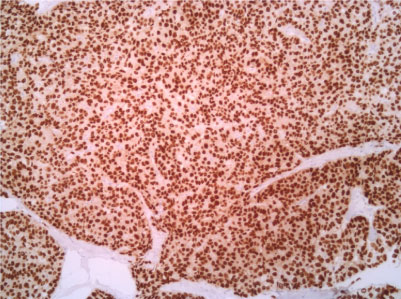 Figure 7: Estrogenic receptor 10X. View Figure 7
Figure 7: Estrogenic receptor 10X. View Figure 7
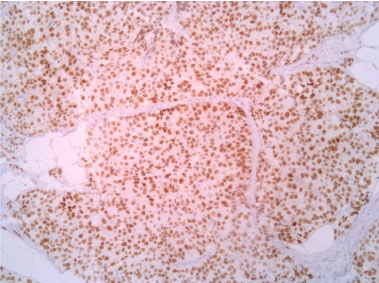 Figure 8: Progestinic receptor 10X. View Figure 8
Figure 8: Progestinic receptor 10X. View Figure 8
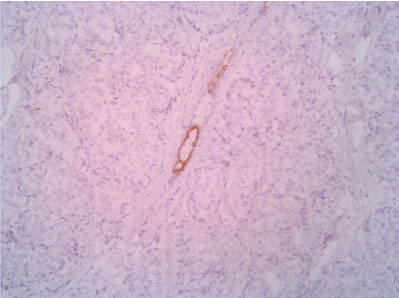 Figure 9: CK5 10X (with positive control - normal duct entrapped in the neoplasm). View Figure 9
Figure 9: CK5 10X (with positive control - normal duct entrapped in the neoplasm). View Figure 9
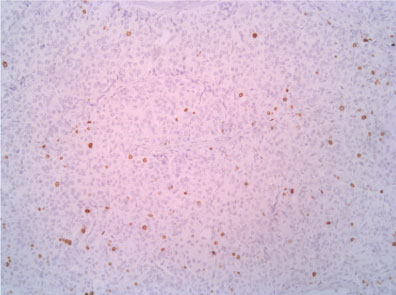 Figure 10: Ki67 10X. View Figure 10
Figure 10: Ki67 10X. View Figure 10
Focal neuroendocrine differentiation could be observed in various histological subtypes of mammary carcinoma [21], including in situ carcinoma and invasive ductal, lobular, colloid, or papillary carcinoma. Primary neuroendocrine cancer of the breast must be distinguished from a metastatic lesion from other sites. Clinical features and morphology are not helpful to distinguish NECB from other subtypes of breast cancer; therefore, immunohistochemistry markers for neuroendocrine differentiation, mainly chromogranin and synaptophysin, should be routinely used to confirm the diagnosis, especially in the cases of mucinous or solid papillary carcinoma in which the suspicion of NECB is high. It is also useful to verify the absence of clinical evidence of a concurrent primary neuroendocrine carcinoma elsewhere. Most published studies about neuroendocrine breast carcinoma emphasize anatomopathological findings, with little reference to imaging results [16-18].
The presence of an in situ ductal carcinoma is a histological evidence that breast is the primary organ of origin [19].
A review of the literature, which rarely records well-differentiated neuroendocrine breast carcinoma, let us conclude that the NECB evolves similarly to the invasive ductal carcinoma and therefore the prognosis depends on the classical criteria of the TNM. The treatment plan may be the same as that of the invasive ductal breast carcinoma, as NECB may later show liver and bone secondarily metastases.
Up to now, our patient is in good general conditions. We have not detected any recurrences of locoregional disease, notwithstanding the presence of bone lesions from plasmacytoma under control by means of specific therapy. Axillary lymph nodes are being tested by ultrasound scan every three months and have not showed pathological changes so far. In conclusion, a well-differentiated neuroendocrine carcinoma is supposed to have a consistent follow-up. It would be useful to perform immunohistochemistry markers even in cases of invasive ductal tumors with in situ component and/or with mucinous component.
There is no conflict of interest with any of the authors involved in this paper.
None.
No ethics approval was needed.
Trovato Agata: Reviewing and editing the article.
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.