The Dutch Uro-Oncology Study Group (DUOS) is a multidisciplinary network of ~30 hospitals involved in research and treatment of urological cancers. We analyzed the influence of treatment at DUOS versus non-DUOS on survival of muscle-invasive bladder cancer (MIBC) patients and explored correlating parameters.
Characteristics of 3472 cT2-4aN0/XM0 MIBC patients who underwent radical cystectomy (RC), with or without neoadjuvant chemotherapy (NAC), were collected by the Netherlands Cancer Registry (NCR). 5-year overall survival (OS) was estimated by the Kaplan-Meier method. Cox regression analyses were performed to determine hazard ratios for pre-defined variables.
5-year OS differed 3.2% in favor of DUOS centers (49.3% vs. 46.1%, p = 0.09). Best survival was observed in patients treated with NAC and RC at DUOS centers (5-year OS 57%). This was 61.1% in cT3-4 patients treated at DUOS centers. NAC was only significantly associated with improved survival in cT3-4a patients treated at DUOS centers (p = 0.0002). Positive surgical margins were less frequent (p = 0.02) and more pelvic lymph nodes (LNs) were collected and identified (p = 0.001) at DUOS centers. Surgical margins, number of identified LNs, and number of positive LNs significantly correlated with OS.
We identified a greater survival benefit by the use of NAC, a higher number of LNs identified, a lower rate of positive surgical margins and a trend towards survival benefit in patients treated at centers that collaborate in the multidisciplinary DUOS national network.
Our retrospective analysis based on 3472 muscle-invasive bladder cancer patients, showed a non-significant trend towards survival benefit when treated in hospitals involved in a national study-group network (DUOS), with significantly superior outcomes concerning neo-adjuvant chemotherapy, surgical margins and lymph node dissection. These factors significantly correlated with an improved survival, favoring treatment at centers that are involved in a multidisciplinary national network with dedicated care for bladder cancer.
Dedicated center, Muscle-invasive bladder cancer, Retrospective cohort analysis, Survival, Multidisciplinary network
Bladder cancer ranks as the ninth most frequently diagnosed cancer worldwide [1]. In the Netherlands, the annual incidence is ~7000 cases of whom 28% have muscle-invasive bladder cancer (MIBC) [2]. Variation in the clinical management of MIBC has been reported on an (inter-)national level [3,4] and may impact survival outcomes. In addition, the dedication of multidisciplinary teams in hospitals, which may be consistent with the volume of patients representing MIBC, may play an important role. Dedication to patient care is almost universally associated with the interest to take part in clinical research [5].
Since professionals, patients organizations, and health insurances become more and more interested in the potential merits of centralization of cancer treatment, medical societies need to address such questions. Minimum standards in cancer care have been developed by 'SONCOS', a Dutch Cancer Committee in which all medical, paramedical, and nursing disciplines involved in cancer care are represented [6].
In the Netherlands, dedicated centers in the management and research of urological cancers collaborate in the Dutch Uro-Oncology Study Group (DUOS), which represents a foundation of multidisciplinary uro-oncological teams at approximately 30 hospitals. DUOS stands for collaboration, high quality care, participation in and initiation of research, as well as provision of information to health care professionals and patients. DUOS represents all eight academic hospitals, The Netherlands Cancer Institute and a sizeable part of the supraregional hospitals. Participation in recent clinical trials, including pivotal studies with the novel check point inhibitors i.e., was exclusively carried out at DUOS centers [7-9]. In the present study, we retrieved data from the Netherlands Cancer Registry (NCR) [3] with the primary aim to compare clinical outcomes of MIBC patients who underwent RC with or without NAC at DUOS versus non-DUOS centers. For this purpose we conducted univariate analysis. In case that would reveal outcome differences, we planned to perform multivariable analysis.
We conducted a nationwide, retrospective, population-based study on patients with cT2-4aN0M0 MIBC from the NCR who underwent RC with curative intent between 2005 and 2014 (data including follow-up data were available for this period). The NCR is a national database in which all newly diagnosed malignancies are registered. Notification is obtained from the registry of histopathology and cytopathology (PALGA) and the National Registry of Hospital Discharge Diagnosis [10]. Independent trained data managers of NCR collected the data on predefined patient, tumor, and treatment characteristics from the 10 files in the hospitals. Follow-up on vital status was censored at 31-1-2017. Topography and morphology are classified according to the International Classification of Diseases for Oncology (ICD-O) and tumor stage according to the TNM classification system [11,12].
The population was stratified according to treatment at DUOS versus non-DUOS centers. We defined a medical center as a DUOS center when that center was an actual member of DUOS between 2011 and 2014. To avoid potential bias by low volume surgical procedures, we chose a minimum surgical volume of 10 RC procedures annually, based on the applicable minimum standard Fby SONCOS criteria in 2012-2014 [13] . Centers (DUOS and non-DUOS) that did not fulfill this criterion were excluded from the analyses.
The following pre-defined variables were retrieved; Age, gender, year of diagnosis, NAC, number of identified LNs, number of positive LNs, surgical margins status, tumor grade, pathological (y)pTNM stage, and 30-day postoperative mortality. We chose to define resected LNs being reported by the pathologist, as 'identified LNs' and subdivided the number of identified LNs in '1-9', '≥10' or 'numbers dissected/counted not documented' [14-16]. The primary end point of the study was the 5-year OS.
The patient and tumor characteristics were compared by chi-square tests for categorical variables. OS for the entire population and subgroups was analyzed by the Kaplan-Meier method. The difference between the survival curves of the subgroups was tested using the Log-rank. To determine the effect of being treated at a DUOS center on OS, we first performed univariate Cox-regression analysis for the entire cohort and per stage group (cT2 vs. cT3-4a). Thereafter, we added step by step patient characteristics (age, gender, year of diagnosis), NAC and post-operative characteristics (tumor grade, number of identified LNs, number of positive LNs, surgical margins status) to the Cox-regression models to analyze the effect of adjustment for these factors on the effect of being treated at a DUOS center on OS. Due to multicollinearity of NAC and (y)pT stage, we chose to only include NAC into the multivariable Cox regression analyses. Statistical analyses were performed with SAS, version 9.4. P-values < 0.05 were considered statistically significant (two-sided testing).
The entire cohort consisted of 3472 patients of whom 82% had cT2 and 18% had cT3-4a disease. Most baseline patient and tumor characteristics (Table S1) were equally distributed between DUOS and non-DUOS centers, but the majority of the population (2583 patients, 74%) had been treated at a DUOS center. Median follow-up was 33 months.
Table S1: Patient and tumor characteristics at moment of diagnosis. View Table S1
There was a modest non-significant difference for the entire cohort in 5-year OS in patients treated at DUOS vs. non-DUOS centers (49.3% (95% CI 47.3 - 51.4) vs. 46.1% (95% CI 42.6 - 49.5), p = 0.09)) (Figure 1A). Patients treated with NAC and RC at DUOS centers (n = 242) had the best outcome (5-year OS 57.0% (95% CI: 49.5-63.8)), whereas patients not treated at DUOS centers by RC without NAC (n = 809) had the worst outcome (5-year OS 45.8% (95% CI: 42.1-49.3)) (p = 0.001) (Figure 1B).
 Figure 1A: Overall survival following treatment at DUOS versus non-DUOS centers (total group). View Figure 1A
Figure 1A: Overall survival following treatment at DUOS versus non-DUOS centers (total group). View Figure 1A
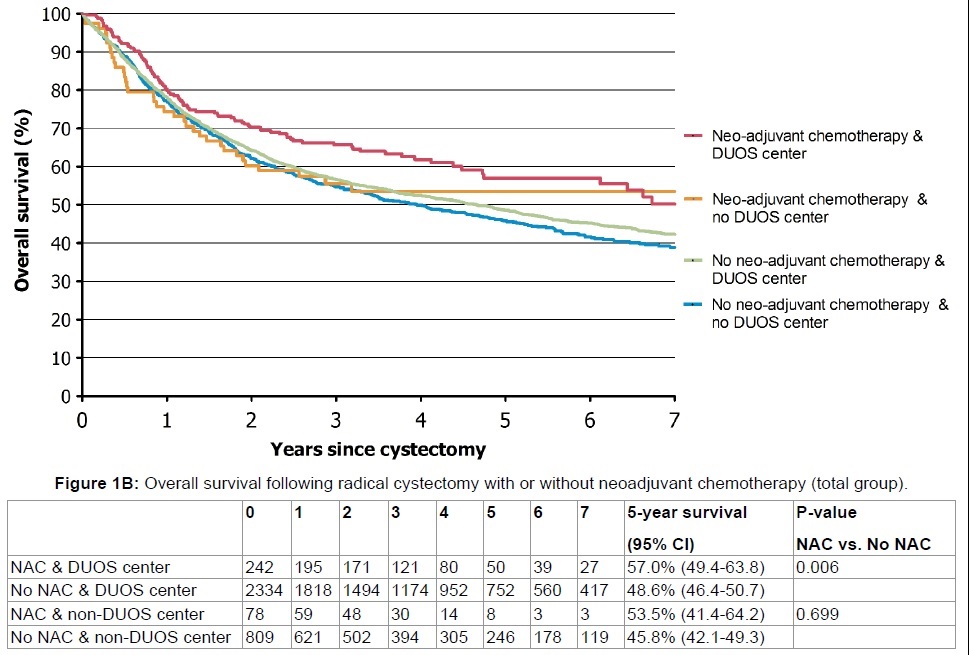 Figure 1B: Overall survival following radical cystectomy with or without neoadjuvant chemotherapy (total group). View Figure 1B
Figure 1B: Overall survival following radical cystectomy with or without neoadjuvant chemotherapy (total group). View Figure 1B
Of all patients, 9.2% received NAC of whom 40% had cT3-4a disease. In later years (2011-2014), the frequency of the use of NAC increased to an average of 25% . The association of receiving NAC with survival was only significant in patients treated at DUOS centers (p = 0.006) (Figure 1B). This retained its significance in cT3-4a patients, after dividing the population into patients with organ-confined (cT2) versus extravesical disease (cT3-4a) (p = 0.0002) (Figure 2A and Figure 2B). Survival at non-DUOS centers did not significantly differ between patients treated with or without NAC (Figure 1B, Figure 2A and Figure 2B).
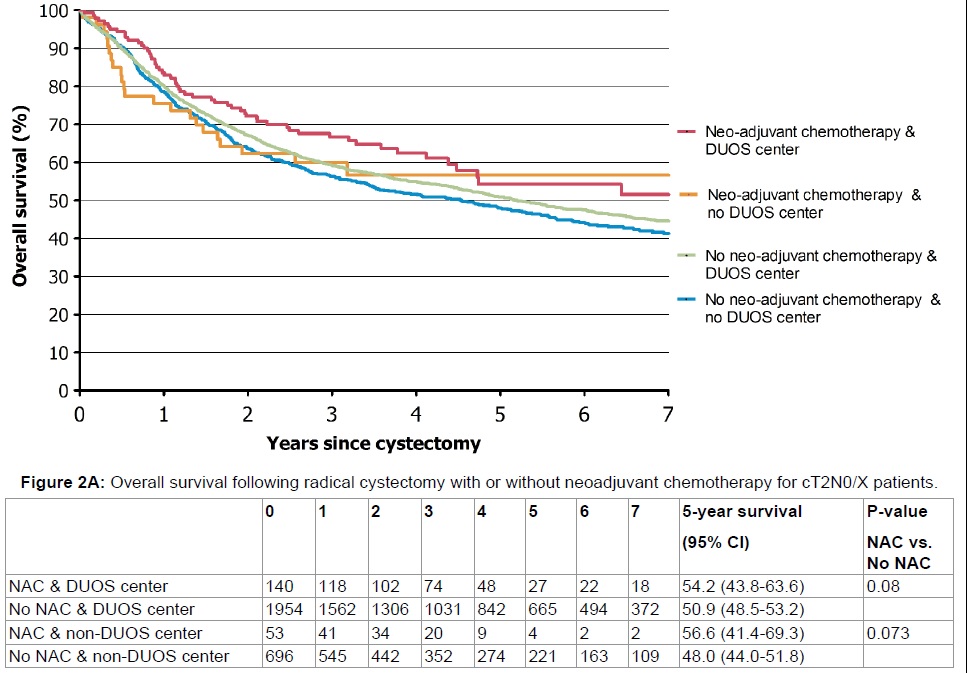 Figure 2A: Overall survival following radical cystectomy with or without neoadjuvant chemotherapy for cT2N0/X patients. View Figure 2A
Figure 2A: Overall survival following radical cystectomy with or without neoadjuvant chemotherapy for cT2N0/X patients. View Figure 2A
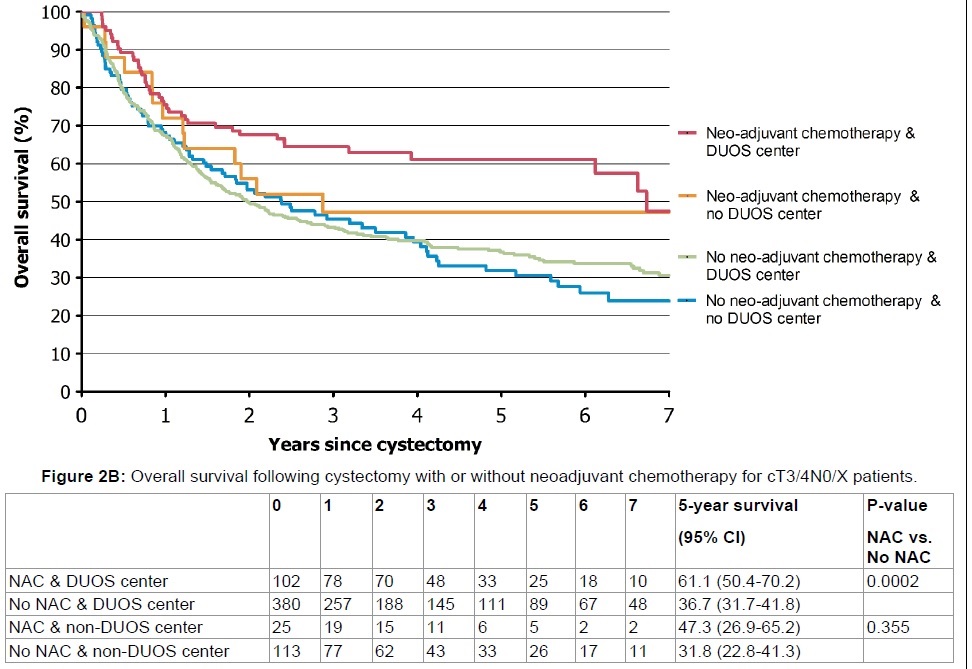 Figure 2B: Overall survival following cystectomy with or without neoadjuvant chemotherapy for cT3/4N0/X patients. View Figure 2B
Figure 2B: Overall survival following cystectomy with or without neoadjuvant chemotherapy for cT3/4N0/X patients. View Figure 2B
After stratifying the population into cT2 and cT3-4a patients, the 5-year OS in cT2 patients who were treated with NAC and RC did not differ between type of center 54.2% (95% CI: 43.8-63.6) vs. 56.6% (95% CI: 41.4-69.3, p = 0.36) (Figure 2A). Patients treated with NAC and RC at DUOS centers had a 6.2% superior survival compared to RC without NAC at non-DUOS centers (p = 0.02) (Figure 2A). In cT3-4 patients treated with NAC and RC, the 5-year OS differed 13.8% (DUOS: 61.1% (95% CI: 50.4 - 70.2) versus non-DUOS: 47.3% (95% CI: 26.9 - 65.2)), which did not reach statistical significance (p = 0.26) (Figure 2B). In cT3-4a patients treated with RC and NAC at DUOS centers, the 5-year OS was 61.1% (95% CI: 50.4 - 70.2) versus 31.8% (95% CI: 22.8-41.3)) for cT3-T4a patients who had undergone RC without NAC at non-DUOS centers (p < 0.001) (Figure 2B).
In patients treated at DUOS centers, significantly more LNs were identified (P < 0.0001) (Table 1). In a larger percentage of patients at non-DUOS centers, the number of dissected or counted LNs was unavailable of not specified (22% vs. 16%, p < 0.001) as well as lacking documentation about positive LNs (12% vs. 6% of patients). There was a small, but significant, difference in the presence of positive surgical margins (9% vs. 7%) in favor of treatment at DUOS centers (p = 0.02).
Table 1: Histopathological characteristics of the radical cystectomy specimen and the 30-day mortality rate (total group). View Table 1
Univariate Cox regression analysis (Table 2) showed a small positive, but non-significant association of being treated at a DUOS center on OS. The step by step addition of patient characteristics (model 2), NAC (model 3), and (post)-operative factors to the Cox-regression multivariable model had little effect on the hazard ratio of being treated at a DUOS center. In the final model, the type of center still had a small non-significant beneficial association with OS. The multivariable Cox-regression (model 4) showed a significant effect for age, the number of identified LNs, the number of positive LNs, and surgical margins status in the total group and both stage groups. In addition, NAC showed only a positive effect (p = 0.04, HR: 0.72, 95% CI: 0.53-0.99) in the cT3-4 cohort. Lacking documentation on the number of identified or positive LNs was negatively associated with survival.
Table 2: Cox-regression on overall survival. View Table 2
Reported disease-free survival and OS for MIBC patients are still fueling debate on factors that are either hypothetically or more likely associated with survival outcome. Discussions about minimum standards of care and centralization of MIBC management are standard components in these discussions. Also variable use of NAC in clinical practice despite level 1 evidence is part of this discussion [17-22]. For the Dutch cancer population, several factors are being registered in the Netherlands Cancer Registry, which makes it possible to retrospectively look at survival differences in relation to different factors.
When analyzing differences between DUOS and non-DUOS centers, univariate analysis in the present study showed the best outcome for cT2-4a patients treated with NAC and RC at DUOS centers with a 5-year OS of 57%, whereas patients not treated at DUOS centers by RC without NAC had the worst outcome with a 5-year OS of 46%. In the subgroup of 620 patients with extravesical disease (cT3-T4a), this difference were more profound with a ~29% difference in the 5-year OS. Such a difference is not likely to be explained completely by the effect of NAC and selection bias. However, we did identify no significant benefit of NAC for patients treated at non-DUOS centers and advantage of NAC was only seen in cT3-4 patients treated at DUOS centers. This retained significance in multivariable analyses in this population (HR 0.72, p = 0.04), emphasizing its importance. Since the proportion of patients receiving NAC did not appear different in our dataset, other factors such as type of chemotherapy, number of cycles and dose-adherence may have played a role. Also treatment of frail patients is likely associated with management in high-volume centers.
Furthermore, in the univariate analysis there was a significant difference in the extent of the lymph node dissection (LND) and the frequency of negative surgical margins in favor of DUOS centers. These two factors have previously been reported to influence the prognosis following RC [14-16,22,23]. The association between quality of LND and high-volume centers is plausible. Several studies have shown an association between survival outcome and treatment at low- or high volume centers [24-26], all favoring treatment at high volume centers. Whether the difference in numbers of LNs is a consequence of the surgical skills or numeration by the pathologist is probably ambiguous, but Leissner, et al. [14] previously showed in their study that the variance in number of LNs was statistically significantly allocated to the different surgeons and not to the pathologists.
In 2011 a Dutch study on the association between high-volume centers and improved outcomes was published [26]. The authors concluded that an important limit is the substantial difference in defining high- or low-volume centers in the literature. For the Dutch situation, this minimum standard has been up scaled in 2015 from ≥ 10 RCs annually to an average of ≥ 20 annually over a period of 3 years [27]. The reason that we have excluded hospitals with < 10 RCs annually for our analysis is a consequence of the period (2005 - 2014) from which our data originated.
There are several limitations to our study. One is its retrospective nature. Another is the lack of documented comorbidities, including renal function, lack on detailed information on the NAC schemes that have been applied, as well as protocol dose-adherence because these data are not routinely registered in the NCR. Although the use of NAC is nowadays considered standard treatment in the management of MIBC, due to renal function impairment and other comorbidieties in this generally frail patient population, approximately 50% of patients do not receive NAC. In our study , at centers where NAC was considered standard therapy in all MIBC, it was found that also in later years the frequency of NAC was limited to around 50% of patients, presumanbly in the majority of cased due to renal function impairment We found no difference in pathological downstaging between type of center, and the proportion of complete downstaging, which is in accordance with the literature [20,28,29]. The slightly larger difference in survival for patients treated with NAC at DUOS centers as compared with literature [18-20] might be a result from bias by selecting patients fit to receive platinum-based chemotherapy, as well as improved NAC regimens (gemcitabin/cisplatin or dose dense MVAC) over the years.
When comparing centers involved in a multidisciplinary national network (DUOS), we found a statistical significant greater survival benefit by the use of NAC, a significant higher number of lymph nodes identified, and a lower rate of positive surgical margins. There was a non-significant trent towards overall survival benefit.